具有强大抗病毒活性的 SARS-CoV-2 主要蛋白酶非肽不可逆抑制剂
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
SARS-CoV-2 感染对易感患者构成高风险。在这项研究中,我们设计了具有多种取代基的苯甲酸卤吡啶酯作为主要病毒蛋白酶(Mpro)的不可逆抑制剂。我们共合成了 55 种苯甲酰氯/溴吡啶酯,苯甲酰基团上的取代方式变化很大。研究人员采用工作流程进行了多参数优化,包括 SARS-CoV-2 和相关致病冠状病毒的 Mpro 抑制试验、酶抑制持续时间、化合物对谷胱甘肽的稳定性、细胞毒性和抗病毒活性。一些化合物的 IC50 值在低纳摩尔范围内,kinact/Ki 值为 100,000 M-1 s-1,具有很高的抗病毒活性。高分辨率 X 射线共晶体结构表明,正氟苯甲酰基取代起着重要作用,形成的水网络可稳定与抑制剂结合的酶。最有效的抗病毒化合物是对乙氧基-邻氟苯甲酰基氯吡啶酯(PSB-21110,29b,MW 296 g/mol;EC50 2.68 nM),它可作为广谱抗冠状病毒疗法的先导结构。本文章由计算机程序翻译,如有差异,请以英文原文为准。
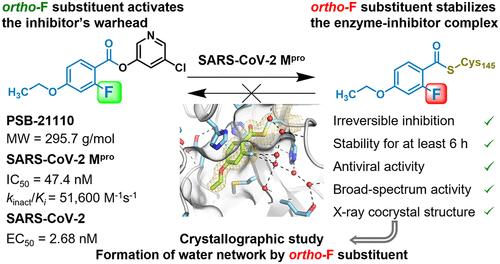
Nonpeptidic Irreversible Inhibitors of SARS-CoV-2 Main Protease with Potent Antiviral Activity
SARS-CoV-2 infections pose a high risk for vulnerable patients. In this study, we designed benzoic acid halopyridyl esters bearing a variety of substituents as irreversible inhibitors of the main viral protease (Mpro). Altogether, 55 benzoyl chloro/bromo-pyridyl esters were synthesized, with broad variation of the substitution pattern on the benzoyl moiety. A workflow was employed for multiparametric optimization, including Mpro inhibition assays of SARS-CoV-2 and related pathogenic coronaviruses, the duration of enzyme inhibition, the compounds’ stability versus glutathione, cytotoxicity, and antiviral activity. Several compounds showed IC50 values in the low nanomolar range, kinact/Ki values of >100,000 M–1 s–1 and high antiviral activity. High-resolution X-ray cocrystal structures indicated an important role of ortho-fluorobenzoyl substitution, forming a water network that stabilizes the inhibitor-bound enzyme. The most potent antiviral compound was the p-ethoxy-o-fluorobenzoyl chloropyridyl ester (PSB-21110, 29b, MW 296 g/mol; EC50 2.68 nM), which may serve as a lead structure for broad-spectrum anticoronaviral therapeutics.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


