利用镍催化和阴离子配体克服非活化烯烃交叉偶联的局限性
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
涉及烯类的多组分交叉偶联反应是获得三维分子的一个引人注目的策略,也是当代药物化学的一个重要追求。过渡金属催化的过程主要需要使用共轭烯烃或带有特定辅助官能团(如 8-氨基喹啉)的非活化烯烃。然而,直接使用未修饰的原生官能团(如醇和醚)作为导向基团仍然是一个巨大的挑战。在这里,我们利用阴离子双齿配体(如 acac),成功地解决了在镍催化的非活化烯烃交叉偶联反应中使用弱配位原生官能团作为指导基团的难题。该反应能在烯烃的两个碳上同时引入一个 sp2 片段和一个 sp3 片段,并具有很高的化学和区域选择性。这项工作证明了阴离子双齿配体在非活化烯烃交叉偶联反应中的优势和潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
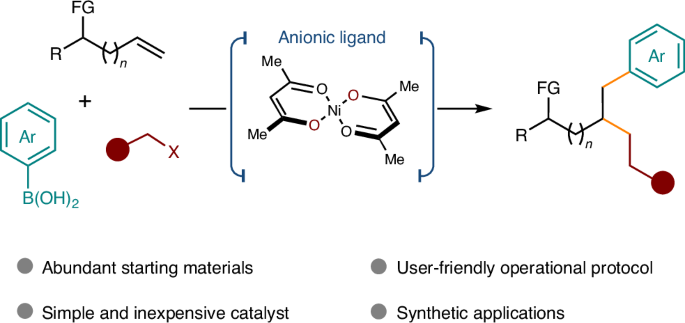
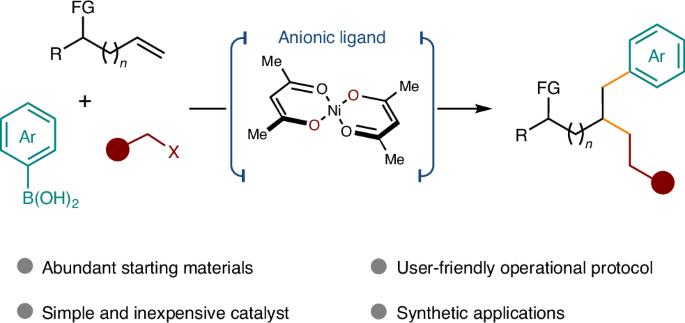
Overcoming limitations in non-activated alkene cross-coupling with nickel catalysis and anionic ligands
Multicomponent cross-coupling reactions involving alkenes represent a compelling strategy for accessing three-dimensional molecules, a key pursuit in contemporary medicinal chemistry. Transition metal-catalysed processes predominantly necessitate the use of conjugated alkenes or non-activated alkenes equipped with specific auxiliary functional groups, for example, 8-aminoquinoline. However, it remains a huge challenge to directly use unmodified native functional groups, such as alcohols and ethers, as directing groups. Here, by utilizing an anionic bidentate ligand such as acac, we have successfully addressed the challenge of employing weakly coordinating native functional groups as directing groups in a nickel-catalysed cross-coupling of non-activated alkenes. This reaction enables the simultaneous introduction of an sp2 fragment and an sp3 fragment to two carbons of the alkenes with high chemo- and regioselectivity. This work demonstrates the advantages and potential of anionic bidentate ligands in the cross-coupling of non-activated alkenes. The transition metal-catalysed multicomponent cross-coupling of alkenes currently relies on strongly coordinating groups to direct the reactivity. Now Wu et al. present a nickel catalyst that enables the installation of sp2 and sp3 fragments on alkenes using alcohols and ethers as directing groups.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


