镍催化的芳基碘化物与杂芳香族硫醚通过 C-S 键裂解的脱硫交叉偶联。
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
Journal of Organic Chemistry
Pub Date : 2024-09-06
Epub Date: 2024-08-15
DOI:10.1021/acs.joc.4c00678
引用次数: 0
摘要
在此,我们介绍了一种镍催化的杂芳香族硫醚与芳基碘化物在还原条件下通过选择性 C(sp2)-S 键裂解直接交叉偶联的方法,从而高效地提供了各种双芳基框架。机理研究表明,Mo(CO)6 在促进 C(sp2)-S 键的活化过程中发挥了关键作用。该方案具有底物范围广、操作简单和官能团兼容性好等特点。此外,该反应的实用性还体现在对杂芳基框架的简单放大和连续修饰上。本文章由计算机程序翻译,如有差异,请以英文原文为准。
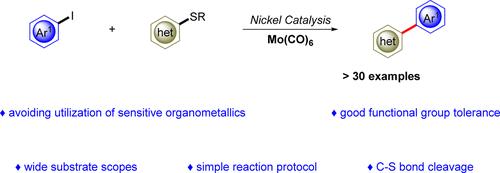
Nickel-Catalyzed Desulfurative Cross-Coupling of Aryl Iodides with Heteroaromatic Thioethers via C-S Bond Cleavage.
Herein, we present a Ni-catalyzed direct cross-coupling of heteroaromatic thioethers with aryl iodides via selective C(sp2)-S bond cleavage under reductive conditions, thereby providing various biaryl frameworks with high efficiency. Mechanistic studies suggested Mo(CO)6 played a crucial role in facilitating the activation of the C(sp2)-S bond. This protocol demonstrated a wide substrate scope, operational simplicity, and good functional group compatibility. Furthermore, the utility of this reaction was highlighted by facile scale-up and sequential modification of heteroaryl frameworks.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


