结构引导下的短链脱氢酶 LfSDR1 工程,用于高效生物合成替诺福韦的关键中间体 (R)-9-(2-Hydroxypropyl)adenine
IF 4.4
2区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
摘要
(<i>R</i>)-9-(2-羟基丙基)腺嘌呤((<i>R</i>)-HPA)是合成替诺福韦及其原药的重要中间体。本文采用结构引导合理设计短链脱氢酶LfSDR1,以提高其在高底物负载下对映选择性合成(<i>R</i>)-HPA的催化性能。研究人员解决了 LfSDR1 的蛋白形态以及与 NADPH 复合物的晶体结构,并将其用于诱变研究和机理说明。通过结构分析确定了三个残基(G92、E141和V186)为热点,并得到了活性显著提高的变体V186A/G92V和V186A/G92V/E141L。通过对 WT 和变体的分子动力学(MD)模拟,G92V 在结合袋中酶与底物的相互作用中起着关键作用。以表达突变体 LfSDR1-V186A/G92V 的全细胞和枯草芽孢杆菌的葡萄糖脱氢酶 BsGDH 为催化剂,在不使用助溶剂的情况下将 200 g L-1 底物完全转化为 (R)-HPA,ee 为 99.9%,时空产率(STY)高达 800 g L-1 day-1。这项研究加深了对 LfSDR 催化机理的理解,为工业合成 (<i>R</i>)-HPA 提供了一种潜在的生物催化策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
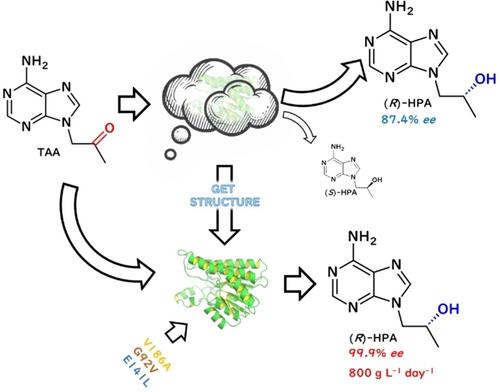
Structure‐Guided Engineering of a Short‐Chain Dehydrogenase LfSDR1 for Efficient Biosynthesis of (R)‐9‐(2‐Hydroxypropyl)adenine, the Key Intermediate of Tenofovir
(R)‐9‐(2‐hydroxypropyl) adenine ((R)‐HPA) is an important intermediate for the synthesis of tenofovir and its prodrugs. Herein, structure‐guided rational design of short‐chain dehydrogenase LfSDR1 was adopted to improve the catalytic performance for enantioselective synthesis of (R)‐HPA at high substrate loading. The crystal structures of LfSDR1 in its apo form as well as in complex with NADPH were solved, which were used for mutagenesis studies and illustration mechanism. Three residues (G92, E141 and V186) were identified as hotspots by structural analysis, and variants V186A/G92V and V186A/G92V/E141L with remarkably improved activity were obtained. By molecular dynamics (MD) simulation of WT and variants, G92V plays a key role in enzyme‐substrate interaction in the binding pocket. Whole cells expressing the mutant LfSDR1‐V186A/G92V and glucose dehydrogenase BsGDH from Bacillus subtilis were used as the catalyst, and up to 200 g L−1 substrate without cosolvent was completely converted to (R)‐HPA with 99.9% ee and a high space‐time yield (STY) of 800 g L−1 day−1. This study improves the understanding of the catalytic mechanism of LfSDR and provides a potential biocatalytic strategy for industrial synthesis of (R)‐HPA.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Advanced Synthesis & Catalysis
化学-应用化学
CiteScore
9.40
自引率
7.40%
发文量
447
审稿时长
1.8 months
期刊介绍:
Advanced Synthesis & Catalysis (ASC) is the leading primary journal in organic, organometallic, and applied chemistry.
The high impact of ASC can be attributed to the unique focus of the journal, which publishes exciting new results from academic and industrial labs on efficient, practical, and environmentally friendly organic synthesis. While homogeneous, heterogeneous, organic, and enzyme catalysis are key technologies to achieve green synthesis, significant contributions to the same goal by synthesis design, reaction techniques, flow chemistry, and continuous processing, multiphase catalysis, green solvents, catalyst immobilization, and recycling, separation science, and process development are also featured in ASC. The Aims and Scope can be found in the Notice to Authors or on the first page of the table of contents in every issue.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


