细菌通过诱导细胞外囊泡的产生来清除有害代谢物,从而调节微藻的衰老生理机能
IF 20.5
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
摘要
水生系统中微藻类的盛衰模式对全球生物地球化学循环有重大贡献。藻华衰退的主要原因是营养限制导致细胞死亡、细胞分裂停止和存活细胞衰老。养分摄入可以重新启动增殖,但对其中的过程却知之甚少。在这里,我们描述了形成藻华的硅藻 Coscinodiscus radiatus 如何在营养流入后从饥饿中恢复。衰老是由细胞外囊泡介导的,这些囊泡将活性氧、氧脂蛋白和其他有害代谢物从老细胞中穿梭出来,从而使细胞重新增殖。通过对衰老细胞施加营养脉冲并对反应进行代谢组学监测,我们发现辐射鳕鱼的调节途径以蛋氨酸循环为中心。共孵育实验表明,细菌利用化学信号介导衰老过程并触发囊泡的产生。这项工作为复杂微生物群落中的细胞衰老和恢复活力开辟了新的视角。本文章由计算机程序翻译,如有差异,请以英文原文为准。
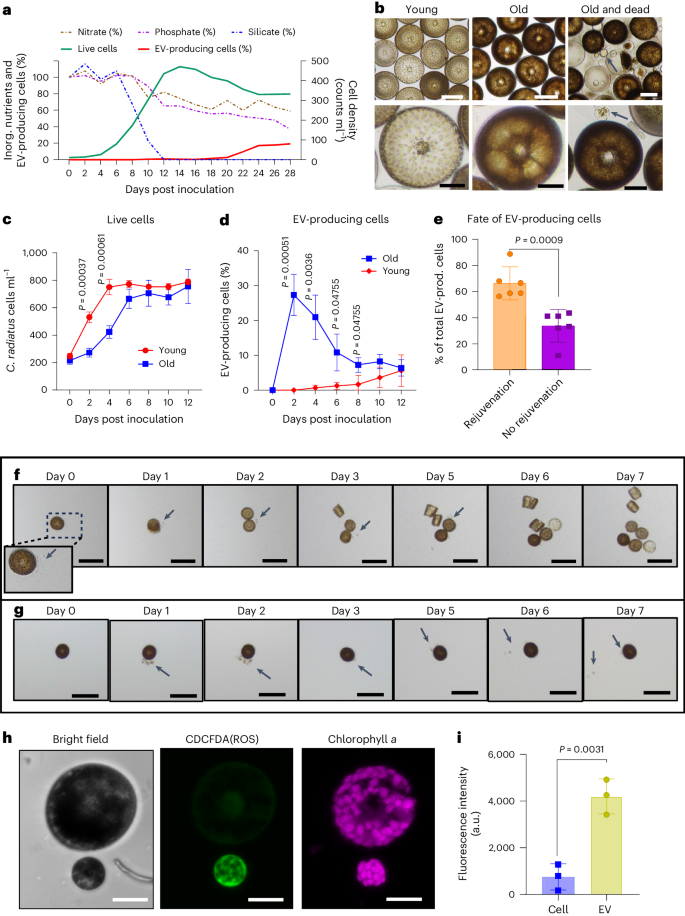
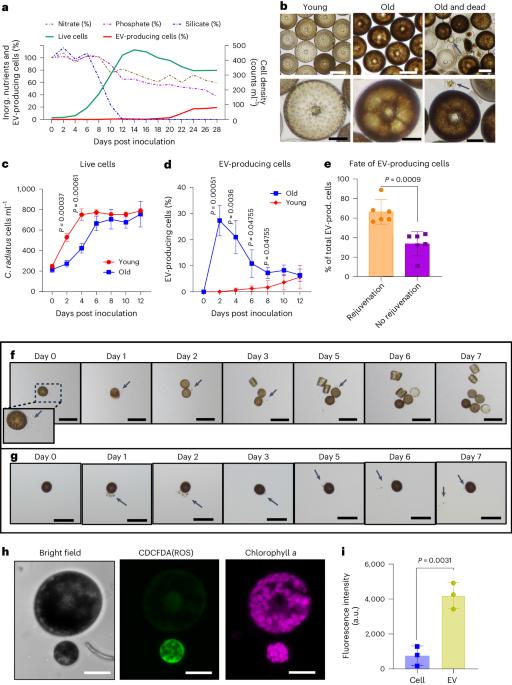
Bacteria modulate microalgal aging physiology through the induction of extracellular vesicle production to remove harmful metabolites
The bloom and bust patterns of microalgae in aquatic systems contribute massively to global biogeochemical cycles. The decline of algal blooms is mainly caused by nutrient limitation resulting in cell death, the arrest of cell division and the aging of surviving cells. Nutrient intake can re-initiate proliferation, but the processes involved are poorly understood. Here we characterize how the bloom-forming diatom Coscinodiscus radiatus recovers from starvation after nutrient influx. Rejuvenation is mediated by extracellular vesicles that shuttle reactive oxygen species, oxylipins and other harmful metabolites out of the old cells, thereby re-enabling their proliferation. By administering nutrient pulses to aged cells and metabolomic monitoring of the response, we show that regulated pathways are centred around the methionine cycle in C. radiatus. Co-incubation experiments show that bacteria mediate aging processes and trigger vesicle production using chemical signalling. This work opens new perspectives on cellular aging and rejuvenation in complex microbial communities. The release of vesicles that shuttle harmful metabolites out of aging cells of the bloom-forming Coscinodiscus radiatus is modulated by bacteria.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Microbiology
Immunology and Microbiology-Microbiology
CiteScore
44.40
自引率
1.10%
发文量
226
期刊介绍:
Nature Microbiology aims to cover a comprehensive range of topics related to microorganisms. This includes:
Evolution: The journal is interested in exploring the evolutionary aspects of microorganisms. This may include research on their genetic diversity, adaptation, and speciation over time.
Physiology and cell biology: Nature Microbiology seeks to understand the functions and characteristics of microorganisms at the cellular and physiological levels. This may involve studying their metabolism, growth patterns, and cellular processes.
Interactions: The journal focuses on the interactions microorganisms have with each other, as well as their interactions with hosts or the environment. This encompasses investigations into microbial communities, symbiotic relationships, and microbial responses to different environments.
Societal significance: Nature Microbiology recognizes the societal impact of microorganisms and welcomes studies that explore their practical applications. This may include research on microbial diseases, biotechnology, or environmental remediation.
In summary, Nature Microbiology is interested in research related to the evolution, physiology and cell biology of microorganisms, their interactions, and their societal relevance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


