实体瘤全基因组测序在精准肿瘤学中的临床应用。
IF 9.5
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
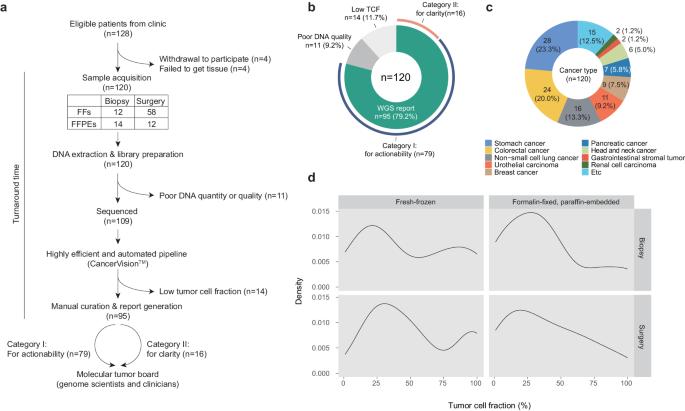
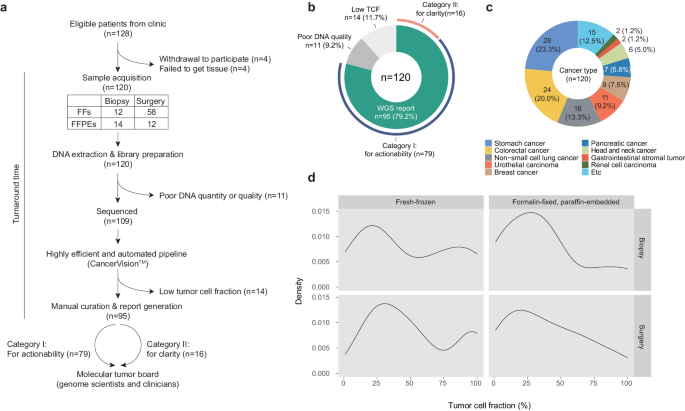
肿瘤的基因组改变在决定肿瘤的临床轨迹和对治疗的反应性方面起着关键作用。在过去十年中,靶向基因组测序(TPS)已成为一种重要的临床工具,但测序成本和生物信息学的进步已使全基因组测序(WGS)成为一种可行的单一检测方法,可用于临床环境中几乎所有癌症基因组的检测。本文报告了一项探索 WGS(肿瘤和匹配的正常组织)实际临床效用的前瞻性单中心研究的结果,该研究有两个主要目标:(1) 评估治疗方案的可操作性;(2) 阐明临床问题。在入组的 120 名各种实体癌患者中,有 95 人(79%)在从取样到报告的中位数 11 个工作日内成功收到了基因组报告。对这 95 份 WGS 报告的分析表明,72%(68/95)的报告具有临床相关性,其中 69%(55/79)与治疗可操作性有关,81%(13/16)与临床清晰度有关。这些益处包括:根据驱动突变、肿瘤突变负荷 (TMB) 和突变特征、需要进行遗传咨询的致病性种系变异以及有助于推断癌症起源的信息的鉴定,选择明智的治疗方法和/或进行积极的临床试验。我们的研究结果凸显了 WGS 作为精准肿瘤学综合工具的潜力,并建议将其纳入常规临床实践,以提供完整的基因组图谱,从而实现量身定制的癌症管理。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Clinical application of whole-genome sequencing of solid tumors for precision oncology
Genomic alterations in tumors play a pivotal role in determining their clinical trajectory and responsiveness to treatment. Targeted panel sequencing (TPS) has served as a key clinical tool over the past decade, but advancements in sequencing costs and bioinformatics have now made whole-genome sequencing (WGS) a feasible single-assay approach for almost all cancer genomes in clinical settings. This paper reports on the findings of a prospective, single-center study exploring the real-world clinical utility of WGS (tumor and matched normal tissues) and has two primary objectives: (1) assessing actionability for therapeutic options and (2) providing clarity for clinical questions. Of the 120 patients with various solid cancers who were enrolled, 95 (79%) successfully received genomic reports within a median of 11 working days from sampling to reporting. Analysis of these 95 WGS reports revealed that 72% (68/95) yielded clinically relevant insights, with 69% (55/79) pertaining to therapeutic actionability and 81% (13/16) pertaining to clinical clarity. These benefits include the selection of informed therapeutics and/or active clinical trials based on the identification of driver mutations, tumor mutational burden (TMB) and mutational signatures, pathogenic germline variants that warrant genetic counseling, and information helpful for inferring cancer origin. Our findings highlight the potential of WGS as a comprehensive tool in precision oncology and suggests that it should be integrated into routine clinical practice to provide a complete image of the genomic landscape to enable tailored cancer management. Personalized medicine customizes cancer treatment to each patient, using molecular profiling of tumors to find specific genetic changes that can guide treatment. Despite progress, the practical use of whole-genome sequencing in clinical settings is still not fully explored. This study examines the use of WGS for cancer patients, aiming to make it a regular part of care. The study involved 120 participants with various solid tumors, using the CancerVisionTM for sequencing. Researchers conclude that WGS is a valuable tool in precision oncology, offering insights that can significantly impact treatment strategies. The study marks progress in integration of genomic medicine into clinical practice, showcasing the feasibility and benefits of WGS in a real-world hospital setting. Future research may further establish WGS as a standard part of cancer care, potentially changing how we approach treatment for different tumor types. This summary was initially drafted using artificial intelligence, then revised and fact-checked by the author.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Experimental and Molecular Medicine
医学-生化与分子生物学
CiteScore
19.50
自引率
0.80%
发文量
166
审稿时长
3 months
期刊介绍:
Experimental & Molecular Medicine (EMM) stands as Korea's pioneering biochemistry journal, established in 1964 and rejuvenated in 1996 as an Open Access, fully peer-reviewed international journal. Dedicated to advancing translational research and showcasing recent breakthroughs in the biomedical realm, EMM invites submissions encompassing genetic, molecular, and cellular studies of human physiology and diseases. Emphasizing the correlation between experimental and translational research and enhanced clinical benefits, the journal actively encourages contributions employing specific molecular tools. Welcoming studies that bridge basic discoveries with clinical relevance, alongside articles demonstrating clear in vivo significance and novelty, Experimental & Molecular Medicine proudly serves as an open-access, online-only repository of cutting-edge medical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


