癌细胞中色素丙烯酰胺与蛋白质相互作用的多层化学蛋白质组图谱
IF 20.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
共价化学是一种扩展人类蛋白质组配体的多功能方法。基于活性的蛋白质分析(ABPP)可以通过与广义反应探针的竞争来推断亲电化合物修饰的特定残基。然而,这种残基定向平台能在多大程度上全面评估亲电化合物在细胞中的蛋白质靶标仍不清楚。在这里,我们评估了一种互补的蛋白质定向 ABPP 方法,该方法可识别与炔化手性亲电化合物--立体探针--发生立体选择性反应的蛋白质。通过整合用色啉丙烯酰胺立体配体处理癌细胞的蛋白质和半胱氨酸定向数据,我们发现了大致相关的配体性图谱,并强调了蛋白质大小和含半胱氨酸肽的蛋白型等特征,这些特征解释了每个 ABPP 平台中存在的差距。我们总共鉴定了 300 种结构和功能各异的蛋白质的立体探针结合事件,其中包括立体选择性和位点特异性地破坏 MAD2L1BP 与纺锤体组装检查点复合物相互作用的化合物,这些化合物会导致癌细胞的有丝分裂退出延迟。本文章由计算机程序翻译,如有差异,请以英文原文为准。
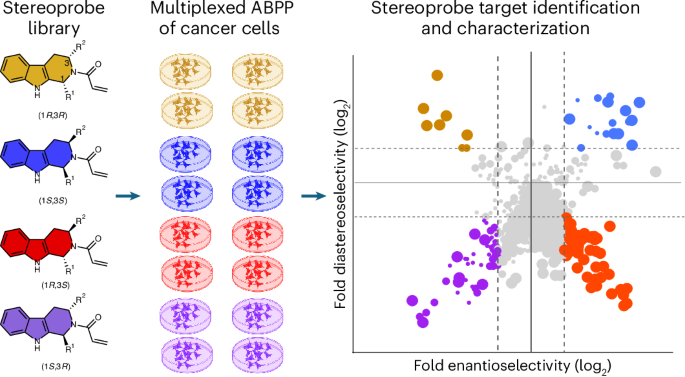
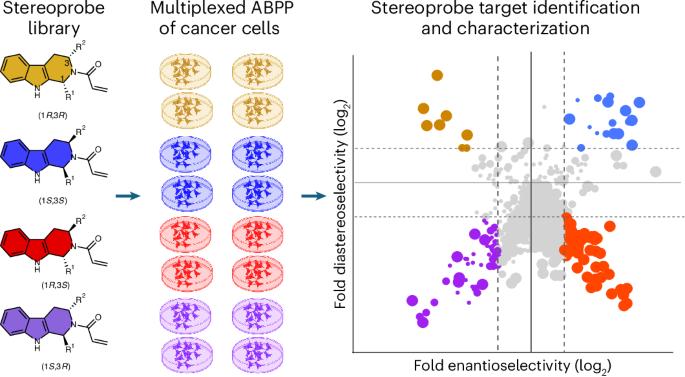
Multi-tiered chemical proteomic maps of tryptoline acrylamide–protein interactions in cancer cells
Covalent chemistry is a versatile approach for expanding the ligandability of the human proteome. Activity-based protein profiling (ABPP) can infer the specific residues modified by electrophilic compounds through competition with broadly reactive probes. However, the extent to which such residue-directed platforms fully assess the protein targets of electrophilic compounds in cells remains unclear. Here we evaluate a complementary protein-directed ABPP method that identifies proteins showing stereoselective reactivity with alkynylated, chiral electrophilic compounds—termed stereoprobes. Integration of protein- and cysteine-directed data from cancer cells treated with tryptoline acrylamide stereoprobes revealed generally well-correlated ligandability maps and highlighted features, such as protein size and the proteotypicity of cysteine-containing peptides, that explain gaps in each ABPP platform. In total, we identified stereoprobe binding events for >300 structurally and functionally diverse proteins, including compounds that stereoselectively and site-specifically disrupt MAD2L1BP interactions with the spindle assembly checkpoint complex leading to delayed mitotic exit in cancer cells. The ligandability of the human proteome can be expanded using covalent chemistry. A multi-tiered chemical proteomic strategy now provides in-depth maps of tryptoline acrylamide–protein interactions in cancer cells. This platform afforded the discovery of stereoselective covalent ligands for hundreds of human proteins, including compounds that disrupt protein–protein interactions regulating the cell cycle.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


