种子辅助形成用于工业规模电流密度阴离子交换膜电解水的镍铁合金阳极催化剂
IF 44.6
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
碱性氧进化反应对于电解水制取绿色氢气至关重要,但在工业要求的安培级电流密度下运行时会遇到巨大挑战,例如传质不足、催化活性降低和寿命有限。在此,我们开发了一种一步种子辅助异质成核法(25 °C,24 小时),用于生产镍-铁基电催化剂(CAPist-L1,CAP 指人工光合作用中心),在 ≥1,000 mA cm-2 的条件下进行强效氧进化反应。基于异质成核系统中的不溶性纳米颗粒,形成了致密的夹层,将催化剂层紧紧固定在基底上,确保了在 1 M KOH 中于 1,000 mA cm-2 下 15,200 小时(21 个月)的长期稳定耐久性。将 CAPist-L1 用作实用阴离子交换膜电解水的阳极催化剂时,它在 2.0 V 电压下的活性高达 7,350 mA cm-2,在 1,000 mA cm-2 温度下的稳定性也很好,在 80 °C 下可持续 1,500 小时。本文章由计算机程序翻译,如有差异,请以英文原文为准。
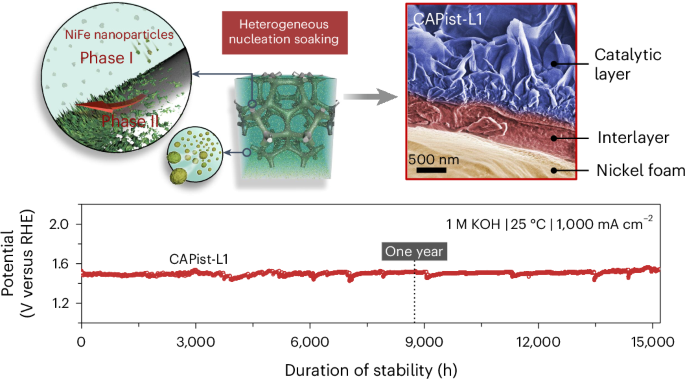
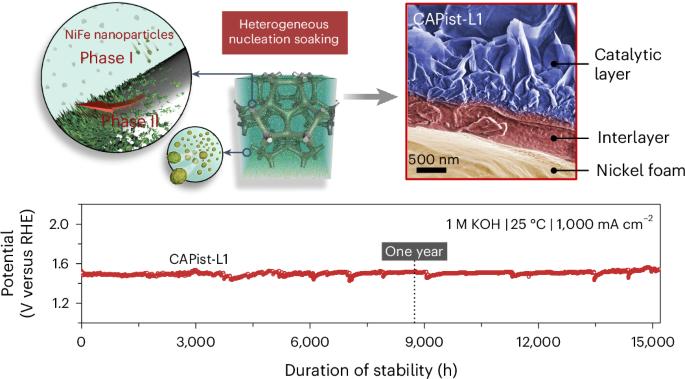
Seed-assisted formation of NiFe anode catalysts for anion exchange membrane water electrolysis at industrial-scale current density
Alkaline oxygen evolution reaction is critical for green hydrogen production from water electrolysis but encounters great challenges when operated at industry-required ampere-scale current densities, such as insufficient mass transfer, reduced catalytic activity and limited lifetimes. Here we develop a one-step seed-assisted heterogeneous nucleation method (25 °C, 24 h) for producing a nickel–iron-based electrocatalyst (CAPist-L1, where CAP refers to the centre of artificial photosynthesis) for robust oxygen evolution reaction at ≥1,000 mA cm−2. Based on the insoluble nanoparticles in the heterogeneous nucleation system, a dense interlayer is formed that anchors the catalyst layer tightly on the substrate, ensuring stable long-term durability of 15,200 h (>21 months) in 1 M KOH at 1,000 mA cm−2. When applying CAPist-L1 as the anode catalyst in practical anion exchange membrane water electrolysis, it delivers a high activity of 7,350 mA cm−2 at 2.0 V and good stability at 1,000 mA cm−2 for 1,500 h at 80 °C. Anion exchange membrane water electrolysis is a promising technology for H2 production using precious metal-free catalysts, but certain hurdles persist for its broad deployment such as the operational stability of its anode catalyst. Now a seed-assisted heterogeneous nucleation method is put forward to prepare a NiFe catalyst with high activity and a stability of over 21 months at 1 A cm−2.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


