强效抗癌剂 4-(4-甲氧基苯基)-3-(3,4,5-三甲氧基苯基)异噁唑的合成和 X 射线结构
IF 1.7
4区 化学
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
以 1,2,3-三甲氧基-5-(硝基甲基)苯和苯甲醚为原料,三步合成了强效抗癌药物 4-(4-甲氧基苯基)-3-(3,4,5-三甲氧基苯基)异噁唑。在表型海胆胚胎试验中,该化合物表现出与天然细胞抑制剂考布他丁 A4(CA4)相当的抗锑蛋白活性。标题异噁唑能抑制 A549 人肺癌细胞和 PC-3 人前列腺癌细胞的体外生长,其 IC50 值分别为 8 纳米和 6 纳米,超过了 CA4 的效果。本文章由计算机程序翻译,如有差异,请以英文原文为准。
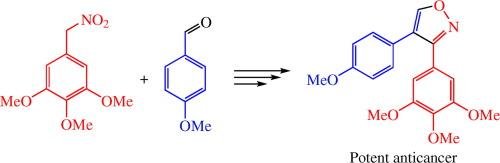
Synthesis and X-ray structure of potent anticancer 4-(4-methoxyphenyl)-3-(3,4,5-trimethoxyphenyl)isoxazole
The three-step synthesis of potent anticancer 4-(4-methoxy- phenyl)-3-(3,4,5-trimethoxyphenyl)isoxazole starting from 1,2,3-trimethoxy-5-(nitromethyl)benzene and anisaldehyde was developed. In phenotypic sea urchin embryo assay, this compound exhibited antimitotic antitubulin activity comparable to that of the natural cytostatic combretastatin A4 (CA4). The title isoxazole inhibited in vitro growth of A549 human lung cancer cells and PC-3 human prostate cancer cells with IC50 values of 8 and 6 nm, respectively, exceeding the effect of CA4.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Mendeleev Communications
化学-化学综合
CiteScore
3.00
自引率
21.10%
发文量
226
审稿时长
4-8 weeks
期刊介绍:
Mendeleev Communications is the journal of the Russian Academy of Sciences, launched jointly by the Academy of Sciences of the USSR and the Royal Society of Chemistry (United Kingdom) in 1991. Starting from 1st January 2007, Elsevier is the new publishing partner of Mendeleev Communications.
Mendeleev Communications publishes short communications in chemistry. The journal primarily features papers from the Russian Federation and the other states of the former USSR. However, it also includes papers by authors from other parts of the world. Mendeleev Communications is not a translated journal, but instead is published directly in English. The International Editorial Board is composed of eminent scientists who provide advice on refereeing policy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


