4-苯基二氮基取代的 3,6-二叔丁基邻苯二酚:合成与偶氮腙平衡
IF 1.8
4区 化学
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
3- 羟基-2,5-二叔丁基-1,4-苯醌与苯肼盐酸盐的反应主要导致位置 1 上相应的腙与 3,6-二叔丁基-4-(苯基-偶氮基)邻苯二酚发生同分异构。核磁共振光谱评估了同分异构的热力学参数。通过 XRD 研究确认了腙结构。本文章由计算机程序翻译,如有差异,请以英文原文为准。
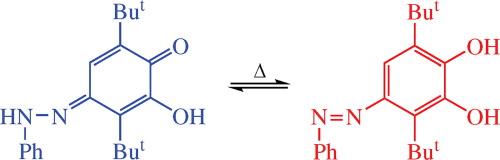
4-Phenyldiazenyl-substituted 3,6-di-tert-butylcatechol: synthesis and azo–hydrazone equilibrium
Reaction of 3-hydroxy-2,5-di-tert-butyl-1,4-benzoquinone with phenylhydrazine hydrochloride mainly leads to the corresponding hydrazone at the position 1 existing in tautomeric equilibrium with 3,6-di-tert-butyl-4-(phenyl- diazenyl)catechol. Thermodynamic parameters of the tautomerization were evaluated by NMR spectroscopy. The hydrazone structure was confirmed by XRD study.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Mendeleev Communications
化学-化学综合
CiteScore
3.00
自引率
21.10%
发文量
226
审稿时长
4-8 weeks
期刊介绍:
Mendeleev Communications is the journal of the Russian Academy of Sciences, launched jointly by the Academy of Sciences of the USSR and the Royal Society of Chemistry (United Kingdom) in 1991. Starting from 1st January 2007, Elsevier is the new publishing partner of Mendeleev Communications.
Mendeleev Communications publishes short communications in chemistry. The journal primarily features papers from the Russian Federation and the other states of the former USSR. However, it also includes papers by authors from other parts of the world. Mendeleev Communications is not a translated journal, but instead is published directly in English. The International Editorial Board is composed of eminent scientists who provide advice on refereeing policy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


