1- 烷基-3-苯基丙炔酮与芳香醛的反应:最新进展
IF 1.8
4区 化学
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
1 烷基-3-苯基丙-2-炔-1-酮在三苯基膦存在下(乙腈,室温,24 小时)与芳香醛顺利发生反应,从而以区域和立体选择性方式得到 (Z)-2-亚苄基氧杂环戊烷-3-酮,收率高达 93%。所提出的转化机理涉及中间体 β-磷酰乙烯基烷基的 1,3-H 质子转移。本文章由计算机程序翻译,如有差异,请以英文原文为准。
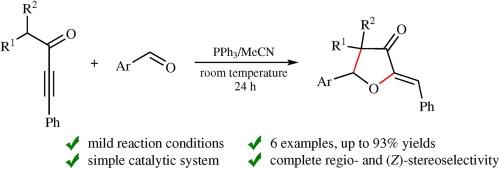
The reaction of 1-alkyl-3-phenylpropynones with aromatic aldehydes: an update
1-Alkyl-3-phenylprop-2-yn-1-ones smoothly react with aromatic aldehydes in the presence of triphenylphosphine (acetonitrile, room temperature, 24 h) to regio- and stereo- selectively afford (Z)-2-benzylideneoxacyclopentan-3-ones in yields up to 93%. The proposed mechanism for the transformation involves 1,3-H proton shift from the alkyl group at the intermediate β-phosphoniovinylide species.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Mendeleev Communications
化学-化学综合
CiteScore
3.00
自引率
21.10%
发文量
226
审稿时长
4-8 weeks
期刊介绍:
Mendeleev Communications is the journal of the Russian Academy of Sciences, launched jointly by the Academy of Sciences of the USSR and the Royal Society of Chemistry (United Kingdom) in 1991. Starting from 1st January 2007, Elsevier is the new publishing partner of Mendeleev Communications.
Mendeleev Communications publishes short communications in chemistry. The journal primarily features papers from the Russian Federation and the other states of the former USSR. However, it also includes papers by authors from other parts of the world. Mendeleev Communications is not a translated journal, but instead is published directly in English. The International Editorial Board is composed of eminent scientists who provide advice on refereeing policy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


