三唑基重氮乙酸酯在光化学条件下的独特反应活性
IF 3.3
Q2 CHEMISTRY, MULTIDISCIPLINARY
ACS Organic & Inorganic Au
Pub Date : 2024-06-12
DOI:10.1021/acsorginorgau.4c0001910.1021/acsorginorgau.4c00019
引用次数: 0
摘要
在光照射下,芳基重氮乙酸酯可根据反应条件生成单重碳化物或三重碳化物,但杂芳基重氮化合物在这方面的研究仍显不足。在这里,我们发现三唑基重氮乙酸酯比芳基重氮乙酸酯具有更高的反应活性。在涉及重氮试剂的光反应中,它们甚至会与常用的惰性溶剂二氯甲烷(DCM)发生反应,生成卤代产物。理论研究表明,所有反应都涉及烯碳化合物,但根据所用溶剂的不同,反应途径也不同。本文章由计算机程序翻译,如有差异,请以英文原文为准。
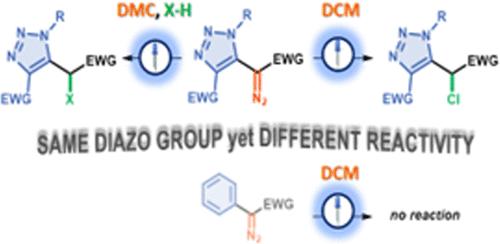
Unique Reactivity of Triazolyl Diazoacetates under Photochemical Conditions
Under light irradiation, aryldiazo acetates can generate either singlet or triplet carbenes depending on the reaction conditions, but heteroaryl diazo compounds have remained underexplored in this context. Herein, we found that triazolyl diazoacetates exhibit higher reactivity than their aryl counterparts. They even react with dichloromethane (DCM), a common, inert solvent, for photoreactions involving diazo reagents, giving halogenated products. Theoretical studies show that all reactions involve carbenes but progress via different pathways depending on the solvent used.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Organic & Inorganic Au
有机化学、无机化学-
CiteScore
4.10
自引率
0.00%
发文量
0
期刊介绍:
ACS Organic & Inorganic Au is an open access journal that publishes original experimental and theoretical/computational studies on organic organometallic inorganic crystal growth and engineering and organic process chemistry. Short letters comprehensive articles reviews and perspectives are welcome on topics that include:Organic chemistry Organometallic chemistry Inorganic Chemistry and Organic Process Chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


