通过螯合辅助 C(sp2)-H 键活化催化喹啉-8-甲醛与 CF3-甲酰亚胺锍酰化物的偶联以合成三氟甲基取代的烯丙胺酮
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
Journal of Organic Chemistry
Pub Date : 2024-07-18
DOI:10.1021/acs.joc.4c0098410.1021/acs.joc.4c00984
引用次数: 0
摘要
本研究开发了一种铑(III)催化的喹啉-8-甲醛与 CF3-亚氨酰基锍酰化物(TFISYs)的 C(sp2)-H 亚氨酰基甲基化醛化反应,用于生成 α-亚氨酰基酮,这些酮很容易以中等到极好的产率同分异构成烯丙酮。在转化过程中,TFISYs 可作为醛基的一种掩蔽烯化试剂,所获得的 CF3 烯酮产物已成功转化为其他有用的三氟甲基取代杂环。本文章由计算机程序翻译,如有差异,请以英文原文为准。
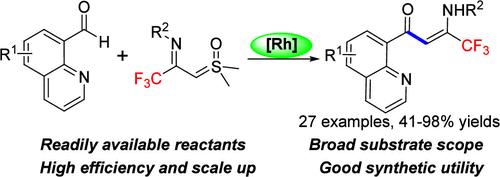
Rhodium(III)-Catalyzed Coupling of Quinolin-8-carboxaldehydes with CF3–Imidoyl Sulfoxonium Ylides by Chelation-Assisted C(sp2)-H Bond Activation for the Synthesis of Trifluoromethyl-Substituted Enaminones
A rhodium(III)-catalyzed aldehydic C(sp2)–H imidoylmethylation of quinolin-8-carboxaldehydes with CF3-imidoyl sulfoxonium ylides (TFISYs) has been developed for the generation of α-imino ketones, which could be readily tautomerized to enaminones in moderate to excellent yields. In the transformation, TFISYs act as a kind of masked alkenylating reagents for the aldehyde moiety, and the obtained CF3-enaminone products have been successfully converted into other useful trifluoromethyl-substituted heterocycles.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


