通过叠氮-乌基反应合成的马来酰亚胺氨基亚胺双四唑抑制癌细胞生长
IF 1.8
4区 化学
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
马来酰亚胺酸甲酯 N-(2-氨基乙基)亚胺与异氰酸酯、多聚甲醛和三甲基硅叠氮化物的叠氮-Ugi 反应一步生成了 1,5 二甲基二萜双四唑。这些化合物对 NCI-60 癌细胞具有选择性细胞毒性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
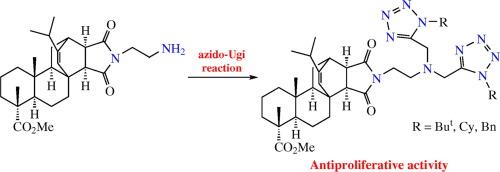
Inhibiting the cancer cell growth by maleopimarate amino imide bis-tetrazoles synthesized via the azido-Ugi reaction
The azido-Ugi reaction of methyl maleopimarate N-(2- aminoethyl) imide with isocyanides, paraformaldehyde and trimethylsilyl azide in one step leads to 1,5-disubstituted diterpene bis-tetrazoles. The compounds demonstrate selective cytotoxicity against NCI-60 cancer cell panel.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Mendeleev Communications
化学-化学综合
CiteScore
3.00
自引率
21.10%
发文量
226
审稿时长
4-8 weeks
期刊介绍:
Mendeleev Communications is the journal of the Russian Academy of Sciences, launched jointly by the Academy of Sciences of the USSR and the Royal Society of Chemistry (United Kingdom) in 1991. Starting from 1st January 2007, Elsevier is the new publishing partner of Mendeleev Communications.
Mendeleev Communications publishes short communications in chemistry. The journal primarily features papers from the Russian Federation and the other states of the former USSR. However, it also includes papers by authors from other parts of the world. Mendeleev Communications is not a translated journal, but instead is published directly in English. The International Editorial Board is composed of eminent scientists who provide advice on refereeing policy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


