铱催化二氧化碳生成氨基甲酸烯丙酯的计算研究
IF 2.9
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
我们采用计算方法研究了铱催化烯丙基置换导致二氧化碳(CO2)形成对映体富集烯丙基氨基甲酸酯的过程。反应分几个步骤进行,首先是铱-烯丙基的形成,然后是二氧化碳和胺在原位形成的氨基甲酸酯的亲核攻击。详细的同分异构体分析表明,(R)- 和 (S) - 途径的速率决定步骤是不同的。这些见解对于理解由 CO2 对映体选择性地形成氨基甲酸烯丙酯的反应至关重要。本文章由计算机程序翻译,如有差异,请以英文原文为准。
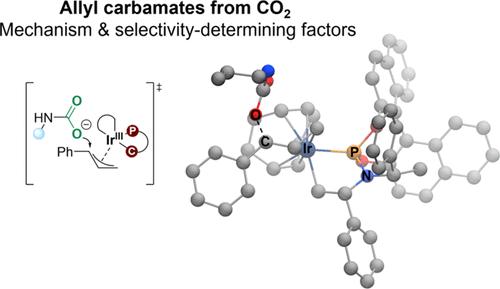
Computational Study of the Ir-Catalyzed Formation of Allyl Carbamates from CO2
We have employed computational methods to investigate the iridium-catalyzed allylic substitution leading to the formation of enantioenriched allyl carbamates from carbon dioxide (CO2). The reaction occurs in several steps, with initial formation of an iridium-allyl, followed by nucleophilic attack by the carbamate formed in situ from CO2 and an amine. A detailed isomeric analysis shows that the rate-determining step differs for the (R)- and (S)-pathways. These insights are essential for understanding reactions involving enantioselective formation of allyl carbamates from CO2.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organometallics
化学-无机化学与核化学
CiteScore
5.60
自引率
7.10%
发文量
382
审稿时长
1.7 months
期刊介绍:
Organometallics is the flagship journal of organometallic chemistry and records progress in one of the most active fields of science, bridging organic and inorganic chemistry. The journal publishes Articles, Communications, Reviews, and Tutorials (instructional overviews) that depict research on the synthesis, structure, bonding, chemical reactivity, and reaction mechanisms for a variety of applications, including catalyst design and catalytic processes; main-group, transition-metal, and lanthanide and actinide metal chemistry; synthetic aspects of polymer science and materials science; and bioorganometallic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


