五甲基环戊二烯三甲基锗:五甲基环戊二烯基三甲基锗:中价单五甲基环戊二烯基氯化钒络合物的无毒入口
IF 2.5
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
VCl3(thf)3]与[Ge(C5Me5)Me3]的反应为钒(III)三聚体[{VCp*(μ-Cl)2}3](Cp* = η5-C5Me5)(1)提供了一种高效、无毒的替代合成方法。1 的三核结构在芳香烃溶剂中得以保留,并且很容易被 [FeCp2](BPh4)氧化,生成 [{VCp*(μ-Cl)2}3](BPh4)。相反,1 在配位溶剂中发生解离,形成单核物种 [VCp*Cl2(L)] (L = thf,py),可与镁还原生成双核混价 V(III)/V(II) [(VCp*)2(μ-Cl)3]或 V(II) [{VCp*(py)(μ-Cl)}2] 衍生物。后一种化合物在室温下以两个电子还原偶氮苯的 N═N键,生成钒(III)衍生物[{VCp*(μ-Cl)}2(μ-η2:η2-N2Ph2)],该衍生物在 90 °C时发生 N-N 键裂解,生成酰亚胺桥接的双核钒(IV)配合物[{VCp*Cl(μ-NPh)}2]。本文章由计算机程序翻译,如有差异,请以英文原文为准。
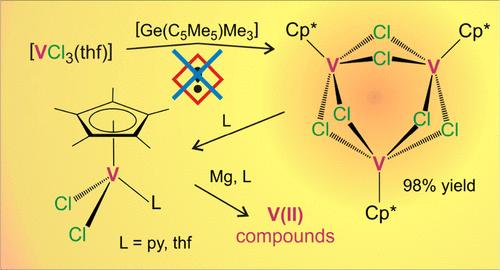
Pentamethylcyclopentadienyltrimethylgermane: A Nontoxic Entry to Mid-Valent Monopentamethylcyclopentadienylvanadium Chloride Complexes
The reaction of [VCl3(thf)3] with [Ge(C5Me5)Me3] provides an efficient and nontoxic alternative synthesis for the vanadium(III) trimer [{VCp*(μ-Cl)2}3] (Cp* = η5-C5Me5) (1). The trinuclear structure of 1 is preserved in aromatic hydrocarbon solvents and can be easily oxidized with [FeCp2](BPh4) to give [{VCp*(μ-Cl)2}3](BPh4). In contrast, 1 undergoes dissociation in coordinating solvents to form mononuclear species [VCp*Cl2(L)] (L = thf, py), which can be reduced with magnesium to afford dinuclear mixed-valence V(III)/V(II) [(VCp*)2(μ-Cl)3] or V(II) [{VCp*(py)(μ-Cl)}2] derivatives. The latter compound reduces in two electrons the N═N bond of azobenzene at room temperature to give the vanadium(III) derivative [{VCp*(μ-Cl)}2(μ–η2:η2-N2Ph2)], which undergoes cleavage of the N–N bond at 90 °C to form the imido-bridged dinuclear vanadium(IV) complex [{VCp*Cl(μ-NPh)}2].
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organometallics
化学-无机化学与核化学
CiteScore
5.60
自引率
7.10%
发文量
382
审稿时长
1.7 months
期刊介绍:
Organometallics is the flagship journal of organometallic chemistry and records progress in one of the most active fields of science, bridging organic and inorganic chemistry. The journal publishes Articles, Communications, Reviews, and Tutorials (instructional overviews) that depict research on the synthesis, structure, bonding, chemical reactivity, and reaction mechanisms for a variety of applications, including catalyst design and catalytic processes; main-group, transition-metal, and lanthanide and actinide metal chemistry; synthetic aspects of polymer science and materials science; and bioorganometallic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


