伊立菲酶在与已故供体交叉配型阳性的高敏肾移植受者中的应用
IF 5.7
2区 医学
Q1 UROLOGY & NEPHROLOGY
引用次数: 0
摘要
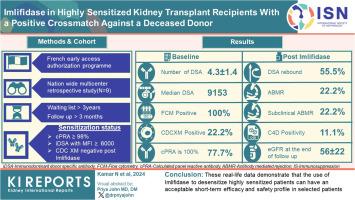
伊立菲酶已获准用于对已故供体交叉配型(XM)阳性的高度致敏成人肾移植候选者进行脱敏治疗。在此,我们报告了在此背景下进行移植的首批 9 名患者的结果,这些患者至少接受了 3 个月的随访。资格标准如下:计算出的全组反应性抗体(cPRA)³ 98%,在等待名单上等待了³ 3 年,平均荧光强度(MFI)大于 6000(1:10 稀释时小于 5000)的免疫优势供体特异性抗体(DSA),以及免疫抑制酶后补体依赖性细胞毒性 XM(CDCXM)阴性。所有9名患者平均透析时间为123±41个月,cPRA为99%(=2)或100%(=7)。移植时,DSA 的平均数量为 4.3 ± 1.4。免疫优势 DSA MFI 中位数为 9153(6430-16980)。在注射伊立菲酶前,分别有 9 名和 2 名患者的流式细胞术 XM(FCXM)和 CDCXM 呈阳性。注射一次伊立菲酶后,所有患者均为阴性。患者接受了多克隆抗体、静脉注射 Igs(IVIg)、利妥昔单抗、他克莫司和霉酚酸酯治疗。五名患者在最初的14天内出现了DSA反弹:两名患者同时出现了临床抗体介导的排斥反应(ABMR),两名患者出现了亚临床ABMR,一名患者出现了孤立的C4d染色阳性。未出现反弹的患者未观察到 ABMR。慢性肾脏病流行病学协作组公式估算的肾小球滤过率(eGFR)在最后一次随访(7 ± 2.8 个月)时为 56 ± 22 ml/min per 1.73 m。未发现移植物丢失或死亡。四名患者至少发生了一次感染。这些真实数据表明,使用伊立菲酶对高度致敏患者进行脱敏治疗,在选定的患者中具有可接受的短期疗效和安全性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Imlifidase in Highly Sensitized Kidney Transplant Recipients With a Positive Crossmatch Against a Deceased Donor
Introduction
Imlifidase is authorized for desensitization of highly sensitized adult kidney transplant candidates with a positive crossmatch (XM) against a deceased donor. Here, we report on the results for the first 9 patients transplanted in this context who had at least 3 months of follow-up.
Methods
The eligibility criteria were as follows: calculated panel reactive antibodies (cPRA) ³ 98%, ³ 3 years on the waiting list, immunodominant donor-specific antibodies (DSAs) with mean fluorescence intensity (MFI) > 6000 (and < 5000 at 1:10 dilution) and a negative post-imlifidase complement-dependent cytotoxic XM (CDCXM).
Results
All 9 patients had been on dialysis for an average of 123 ± 41 months, with cPRA at 99% (n = 2) or 100% (n = 7). At transplantation, the mean number of DSAs was 4.3 ± 1.4. The median immunodominant DSA MFI was 9153 (6430–16,980). Flow cytometry XM (FCXM) and CDCXM before imlifidase were positive in 9 and 2 patients, respectively. After 1 injection of imlifidase, all were negative. Patients received polyclonal antibodies, i.v. Igs (IVIg), rituximab, tacrolimus, and mycophenolate. Five patients had a DSA rebound within the first 14 days: 2 had concomitant clinical antibody-mediated rejection (ABMR), 2 had subclinical ABMR, and 1 had isolated positive C4d staining. No ABMR was observed in patients without rebound. Chronic Kidney Disease-Epidemiology Collaboration formula estimated glomerular filtration rate (eGFR) was 56 ± 22 ml/min per 1.73 m2 at the last follow-up (7 ± 2.8 months). No graft loss or death were observed. Four patients developed at least 1 infection.
Conclusion
These real-life data demonstrate that the use of imlifidase to desensitize highly sensitized patients can have an acceptable short-term efficacy and safety profile in selected patients.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Kidney International Reports
Medicine-Nephrology
CiteScore
7.70
自引率
3.30%
发文量
1578
审稿时长
8 weeks
期刊介绍:
Kidney International Reports, an official journal of the International Society of Nephrology, is a peer-reviewed, open access journal devoted to the publication of leading research and developments related to kidney disease. With the primary aim of contributing to improved care of patients with kidney disease, the journal will publish original clinical and select translational articles and educational content related to the pathogenesis, evaluation and management of acute and chronic kidney disease, end stage renal disease (including transplantation), acid-base, fluid and electrolyte disturbances and hypertension. Of particular interest are submissions related to clinical trials, epidemiology, systematic reviews (including meta-analyses) and outcomes research. The journal will also provide a platform for wider dissemination of national and regional guidelines as well as consensus meeting reports.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


