以氯化胆碱为基础的深共晶溶剂在 293.15 和 313.15 K 温度下进行酒精(正丙醇或正丁醇)-甲酸乙酯酯化的系统中的液液平衡
IF 2.1
3区 工程技术
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
对氯化胆碱和丙二酸或戊二酸形成的深共晶溶剂(DES)进行了分离共沸混合物的测试。为了分离醇酯体系(正丙醇-甲酸乙酯、正丁醇-甲酸乙酯),在 293.15 和 313.15 K 温度和大气压力下,获得了包括 DESs 在内的伪三元体系的液液平衡(LLE)实验数据和相图。利用核磁共振光谱测定并分析了液-液连接线。除了 LLE 数据外,还对所研究的 DES 在 293.15-353.15 K 温度范围内的密度和粘度进行了测量。DESs 的分离性能通过分配系数和对酒精的选择性值来表征。比较了含有醇和酯的体系与氯化胆碱和丙二酸或戊二酸组成的两种 DES 的选择性值。研究发现,所研究的 DES 能够催化酯交换反应,有机相中的转化值取决于醇的浓度。本文章由计算机程序翻译,如有差异,请以英文原文为准。
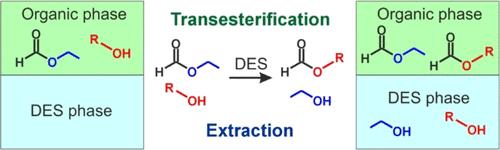
Liquid–Liquid Equilibrium in Systems with Transesterification of Alcohol (n-Propanol Or n-Butanol)–Ethyl Formate with Deep Eutectic Solvent Based on Choline Chloride at 293.15 and 313.15 K
Deep eutectic solvents (DESs) formed by choline chloride and malonic or glutaric acid were tested for separating azeotropic mixtures. To separate alcohol-ester systems (n-propanol–ethyl formate, n-butanol–ethyl formate), the experimental data of liquid-liquid equilibria (LLE) together with phase diagrams for pseudoternary systems including the DESs were obtained at temperatures of 293.15 and 313.15 K and atmospheric pressure. Liquid–liquid tie lines were determined using NMR spectroscopy and analyzed. Along with LLE data, density and viscosity measurements for the studied DESs in the temperature range of 293.15–353.15 K were carried out. The separation performance of DESs was characterized by distribution coefficients and values of selectivity to alcohol. Comparison of selectivity values in systems containing alcohols and esters and two DESs composed of choline chloride and malonic or glutaric acids was performed. It was found that the studied DESs are capable of catalyzing the transesterification reaction, and the conversion values in the organic phase were determined depending on the alcohol concentration.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Chemical & Engineering Data
工程技术-工程:化工
CiteScore
5.20
自引率
19.20%
发文量
324
审稿时长
2.2 months
期刊介绍:
The Journal of Chemical & Engineering Data is a monthly journal devoted to the publication of data obtained from both experiment and computation, which are viewed as complementary. It is the only American Chemical Society journal primarily concerned with articles containing data on the phase behavior and the physical, thermodynamic, and transport properties of well-defined materials, including complex mixtures of known compositions. While environmental and biological samples are of interest, their compositions must be known and reproducible. As a result, adsorption on natural product materials does not generally fit within the scope of Journal of Chemical & Engineering Data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


