基于电位计和电导测量的三元体系(1-己基-3-甲基溴化咪唑鎓+乙醇+水)热力学和传输特性研究
IF 2.1
3区 工程技术
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
本研究采用电导法和电位法分别测定了 1-己基-3-甲基溴化咪唑鎓[HMIm]Br 在[HMIm]Br + 乙醇 + 水三元体系中的摩尔电导率和平均活性系数。在 T = 298.2、308.2 和 308.2 温度条件下,在乙醇 + 水混合物((w/w)% = 湿乙醇/混合物 = 0、10、20 和 30%)中不同质量分数的乙醇上,获得了 0.0008 至 0.2496 mol-kg-1 的[HMIm]Br 离子液体的电导数据。离子结合常数(Ka)和[HMIm]Br 的极限摩尔电导率(Λ0)由 Fuoss-Onsager 方程求得,并利用该方程得到热力学函数。此外,还收集了[HMIm]-ISE 型电偶的电位数据:[HMIm]-ISE|[HMIm]Br(m[HMIm]Br)、乙醇(重量)%、H2O(1 -重量)%|Br-ISE 在水+乙醇混合物(湿乙醇/湿水+乙醇 = 0、10 和 20%)中的离子强度从 0.0094 到 1.利用皮策离子相互作用模型建立了热力学性质模型,并评估了皮策离子相互作用参数 β(0)、β(1) 和 Cφ 的值。然后确定了热力学性质,如平均活性系数、过剩吉布斯自由能、渗透系数和溶剂活性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
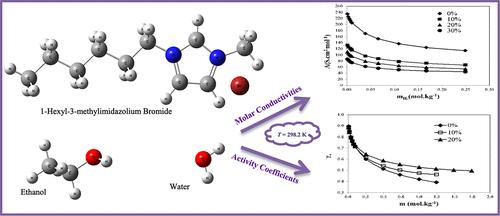
Study of Thermodynamic and Transport Properties of the Ternary System (1-Hexyl-3-Methylimidazolium Bromide + Ethanol + Water) Based on Potentiometric and Conductometric Measurements
In this work, the molar conductivities and mean activity coefficients of 1-hexyl-3- methylimidazolium bromide [HMIm]Br in the ternary system [HMIm]Br + ethanol + water were reported using conductometric and potentiometric techniques, respectively. Conductometric data wereobtained for [HMIm]Br ionic liquids from 0.0008 to 0.2496 mol·kg–1 on various mass fractions of ethanol in ethanol + water mixtures ((w/w)% = wethanol/wmixture = 0, 10, 20, and 30%) at T = 298.2, 308.2, and 318.2 K. Ion association constants (Ka) and limiting molar conductivities (Λ0) of [HMIm]Br were achieved by the Fuoss–Onsager equation and utilized to get the thermodynamic functions. Moreover, potentiometric data were collected for the galvanic cell of the type: [HMIm]–ISE|[HMIm]Br(m[HMIm]Br), ethanol (wt)%, H2O (1 -wt) %|Br–ISE in water + ethanol mixtures (wethanol /wwater+ethanol = 0, 10, and 20%) over the ionic strength varying from 0.0094 to 1.7437 mol·kg–1 at T = 298.2 and 308.2 K. Thermodynamic properties modeling was implemented using the Pitzer ion interaction model and the values of Pitzer ion-interaction parameters β(0), β(1), and Cφ were evaluated. Then, thermodynamic properties, such as mean activity coefficients, excess Gibbs free energy, osmotic coefficients, and solvent activity. were determined.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Chemical & Engineering Data
工程技术-工程:化工
CiteScore
5.20
自引率
19.20%
发文量
324
审稿时长
2.2 months
期刊介绍:
The Journal of Chemical & Engineering Data is a monthly journal devoted to the publication of data obtained from both experiment and computation, which are viewed as complementary. It is the only American Chemical Society journal primarily concerned with articles containing data on the phase behavior and the physical, thermodynamic, and transport properties of well-defined materials, including complex mixtures of known compositions. While environmental and biological samples are of interest, their compositions must be known and reproducible. As a result, adsorption on natural product materials does not generally fit within the scope of Journal of Chemical & Engineering Data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


