莫能吡韦在 15 种单溶剂中的固液平衡:实验与分子模拟研究
IF 2.1
3区 工程技术
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
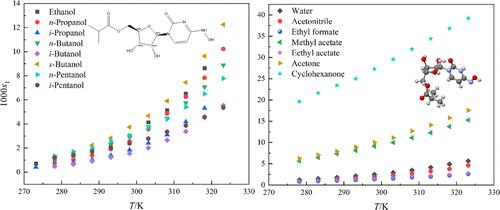
莫能吡韦(MPV)具有很强的抗病毒作用。然而,关于这种药物溶解特性的现有报告很少。本文采用重量法测定了 MPV 在 15 种单溶剂中的溶解度。在所有实验溶剂中,MPV 在环己酮中的溶解度最高,在甲酸乙酯中的溶解度最低。在醇类溶剂中,正丁醇的溶解度最大,而异丁醇的溶解度最小。MPV 的溶解度随着温度的升高而增加。为了拟合 MPV 在 15 种单溶剂中的溶解度数据,选择了包括改进的 Apelblat 模型、λh 模型、Yaws 模型和 Abolghasem Jouyban 模型在内的热力学模型。在所有选定的模型中,Abolghasem Jouyban 模型在 15 种单溶剂中对实验数据的拟合效果最好,相对平均偏差(RAD)= 0.0024。最后,进行了分子动力学模拟、径向分布函数(RDF)分析和密度泛函理论(DFT)计算。结果表明,MPV 可通过范德华相互作用、静电作用和氢键与有机溶剂相互作用。MPV 的实验数据将为优化结晶过程提供重要指导。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Solid–Liquid Equilibrium of Molnupiravir in 15 Monosolvents: An Experimental and Molecular Simulation Study
Molnupiravir (MPV) has a strong antiviral effect. However, there are few existing reports on the solubility properties of this drug. In this article, the solubility of MPV in 15 monosolvents was measured using the gravimetric method. Among all experimental solvents, the solubility of MPV was found to be the highest in cyclohexanone and the lowest in ethyl formate. In alcohol solvents, the maximum solubility was observed in s-butanol, while the minimum value was observed in i-butanol. The solubility of MPV increases with an increase in the temperature. Thermodynamic models including the modified Apelblat model, λh model, Yaws model and Abolghasem Jouyban model were chosen to fit the solubility data in 15 monosolvents. The Abolghasem Jouyban model fitted the experimental data best among all the selected models in 15 monosolvents, with relative average deviation (RAD) = 0.0024. Finally, a molecular dynamics simulation, radial distribution function (RDF) analysis, and density functional theory (DFT) calculation were performed. The results indicated that MPV could interact with organic solvents via van der Waals interactions, electrostatic interactions, and hydrogen bonding. The experimental data for MPV will provide significant guidance for the optimization of the crystallization process.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Chemical & Engineering Data
工程技术-工程:化工
CiteScore
5.20
自引率
19.20%
发文量
324
审稿时长
2.2 months
期刊介绍:
The Journal of Chemical & Engineering Data is a monthly journal devoted to the publication of data obtained from both experiment and computation, which are viewed as complementary. It is the only American Chemical Society journal primarily concerned with articles containing data on the phase behavior and the physical, thermodynamic, and transport properties of well-defined materials, including complex mixtures of known compositions. While environmental and biological samples are of interest, their compositions must be known and reproducible. As a result, adsorption on natural product materials does not generally fit within the scope of Journal of Chemical & Engineering Data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


