通过钯催化脱羧烯丙基烷基化合成 3,3-二取代烯丙基异吲哚啉酮
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在此,我们报告了一种温和的钯催化烯丙基酯取代异吲哚啉酮底物的脱羧烯丙基烷基化反应,从而得到多种 3,3-二取代异吲哚啉酮衍生物。该脱羧偶联反应可耐受一系列官能团,包括酮和烯基卤化物,并且不需要异吲哚啉酮氮的保护。此外,对于大多数评估过的底物,该反应都能以接近定量的收率进行。根据分离出的竞争环丙烷和质子化产物,提出了一种反应机理。本文章由计算机程序翻译,如有差异,请以英文原文为准。
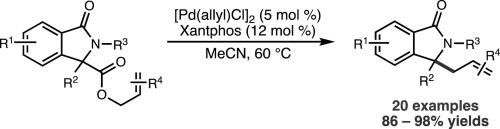
Synthesis of 3,3-disubstituted allyl isoindolinones via Pd-catalyzed decarboxylative allylic alkylation
Herein, we report a mild palladium-catalyzed decarboxylative allylic alkylation of allyl ester-substituted isoindolinone substrates to afford a variety of 3,3-disubstituted isoindolinone derivatives. The decarboxylative coupling reaction tolerates a range of functional groups, including ketones and alkenyl halides, and does not require protection of the isoindolinone nitrogen. Additionally, the reaction was found to proceed in near-quantitative yield for most substrates evaluated. Based on the isolation of competing cyclopropane and protonation products, a reaction mechanism is proposed.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


