合成(S)-托伐普坦和(S)-去甲基托伐普坦的新方法
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们开发了一种新颖简洁的方法来全合成用于治疗低钠血症的()托伐普坦。关键中间体苯并氮杂卓酮的合成分为三个步骤,首先是丙戊酰胺保护芳基胺的酰化反应,然后是分子内卤胺环化反应。以-氯苯胺为原料,通过七个步骤合成了()托伐普坦,总收率为 43%。本文章由计算机程序翻译,如有差异,请以英文原文为准。
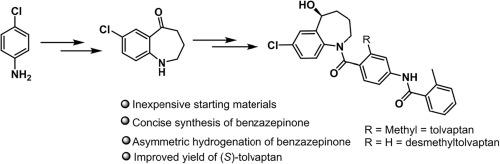
A novel approach for the synthesis of (S)-tolvaptan and (S)-desmethyltolvaptan
A novel and concise approach has been developed for the total synthesis of (S)-tolvaptan, which is used for the treatment of hyponatremia. The key intermediate, benzazepinone has been synthesized in three steps through ortho-acylation of N-Pivalamide protected aryl amine followed by an intramolecular haloamine cyclization. The total synthesis of (S)-tolvaptan from p-chloroaniline has been accomplished in seven steps with 43% overall yield.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


