一种新的齐墩果类三萜苷
IF 0.8
4区 化学
Q4 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
从Nephelium lappaceum L.的种子水乙醇提取物中分离出一种新的齐墩果烷型三萜苷,即3-O-α-L-阿拉伯吡喃糖基-(1→3)-α-L-鼠李糖基-(1→2)-α-L-阿拉伯吡喃糖基齐墩果酸28-O-β-D-木吡喃糖基-(1→3)-β-D-木吡喃糖基-(1→2)-β-D-葡吡喃糖基酯、从 Nephelium lappaceum L. 种子的水乙醇提取物中分离出来。该化合物的结构阐释基于光谱数据分析,包括一维和二维核磁共振以及 HR-ESI-MS 技术,并将其核磁共振数据与文献报道的数据进行了比较。本文章由计算机程序翻译,如有差异,请以英文原文为准。
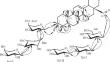

A New Oleanane-Type Triterpene Glycoside from Nephelium lappaceum
A new oleanane-type triterpene glycoside, 3-O-α-L-arabinopyranosyl-(1→3)-α-L-rhamnopyranosyl-(1→2)-α-L-arabinopyranosyloleanolic acid 28-O-β-D-xylopyranosyl-(1→3)-β-D-xylopyranosyl-(1→2)-β-D-glucopyranosyl ester, was isolated from the aqueous-ethanolic extract of the seeds of Nephelium lappaceum L. The structure elucidation of this compound was based on analyses of spectroscopic data, including 1D and 2D NMR and HR-ESI-MS techniques, and by comparing their NMR data with those reported in the literature.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemistry of Natural Compounds
化学-有机化学
CiteScore
1.40
自引率
25.00%
发文量
265
审稿时长
7.8 months
期刊介绍:
Chemistry of Natural Compounds publishes reviews and general articles about the structure of different classes of natural compounds, the chemical characteristics of botanical families, genus, and species, to establish the comparative laws and connection between physiological activity and the structure of substances.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


