甘草酸甲酯新 A-癸基衍生物的合成
IF 0.8
4区 化学
Q4 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
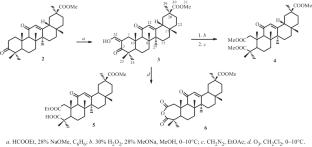
合成了新的甘草酸甲酯(MeGLC)A-共衍生物。在含有 NaOMe(28%)的 MeOH 溶液中,用 H2O2(30%)裂解 MeGLC 的 2-羟基亚甲基-3-氧代衍生物的 A 环,得到 2,3-seco-2,3-二酸,并分离出二甲酯。对 MeGLC 的 2-羟基亚甲基-3-氧代衍生物进行臭氧分解,首次生成了 2,3-seco-2-乙氧羰基-18βH-油-12-烯-2,3,30-三酸甲酯和 2,3-anhydro-11-oxo-18βH-油-9,12-diene-2,3,30-三酸甲酯。本文章由计算机程序翻译,如有差异,请以英文原文为准。
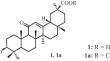
Synthesis of New A-Seco-Derivatives of Methyl Glycyrrhetinate
New A-seco-derivatives of methyl glycyrrhetinate (MeGLC) were synthesized. Cleavage of ring A of the 2-hydroxymethylene-3-oxo-derivative of MeGLC by H2O2 (30%) in the presence of NaOMe (28%) in MeOH led to the 2,3-seco-2,3-diacid, which was isolated as the dimethyl ester. Ozonolysis of the 2-hydroxymethylene-3-oxo-derivative of MeGLC produced for the first time the methyl esters of 2,3-seco-2-ethoxycarbonyl- 18βH-olean-12-ene-2,3,30-trioic acid and 2,3-anhydro-11-oxo-18βH-olean-9,12-diene-2,3,30-trioic acid.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemistry of Natural Compounds
化学-有机化学
CiteScore
1.40
自引率
25.00%
发文量
265
审稿时长
7.8 months
期刊介绍:
Chemistry of Natural Compounds publishes reviews and general articles about the structure of different classes of natural compounds, the chemical characteristics of botanical families, genus, and species, to establish the comparative laws and connection between physiological activity and the structure of substances.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


