具有潜在抗冠状病毒活性的 2-氧甲基胞嘧啶衍生物
IF 0.8
4区 化学
Q4 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
合成了 2-oxomethylcytisine 和 N-取代马来酰亚胺的 Diels-Alder β-endo 加合物。对它们与 SARS-CoV-2 主要蛋白酶 Mpro 的 6LU7 活性位点相互作用的能力进行了硅学评估。测试的化合物包括潜在的抗冠状病毒剂 (3aR,4S,8S,12S,12aS,12bR)-2-(3-甲氧基苯基)-10-甲基-3a,7,8,9,10,12b-六氢-1H-4,12a-乙烯基-8,12-甲烷吡咯并[3′,4′:3,4]吡啶并[1,2-a][1,5]二佐辛-1,3,5,11(2H,4H,12H)-四酮,其计算特性超过了参考配体 tideglusib。本文章由计算机程序翻译,如有差异,请以英文原文为准。
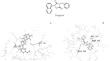
2-Oxomethylcytisine Derivatives with Potential Anti-Coronavirus Activity
Diels−Alder β-endo adducts of 2-oxomethylcytisine and N-substituted maleimides were synthesized. Their ability to interact with the 6LU7 active site of SARS-CoV-2 main protease Mpro was evaluated in silico. The tested compounds included the potential anti-coronavirus agent (3aR,4S,8S,12S,12aS,12bR)-2-(3-methoxyphenyl)-10-methyl-3a,7,8,9,10,12b-hexahydro-1H-4,12a-etheno-8,12-methanopyrrolo[3′,4′:3,4]pyrido[1,2-a][1,5]diazocine-1,3,5,11(2H,4H,12H)-tetrone, which had calculated characteristics exceeding those of the reference ligand tideglusib.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemistry of Natural Compounds
化学-有机化学
CiteScore
1.40
自引率
25.00%
发文量
265
审稿时长
7.8 months
期刊介绍:
Chemistry of Natural Compounds publishes reviews and general articles about the structure of different classes of natural compounds, the chemical characteristics of botanical families, genus, and species, to establish the comparative laws and connection between physiological activity and the structure of substances.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


