通过从 O3 到 O2 结构的相变大幅提高钴酸锂的高压循环性能
IF 11.1
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
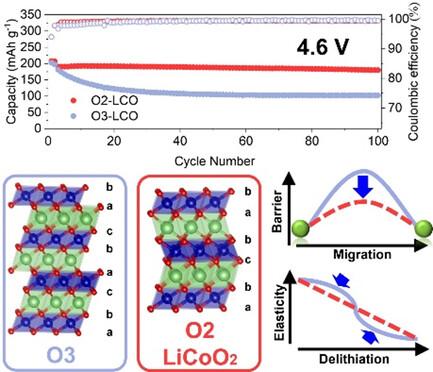
钴酸锂(LiCoO2)是锂离子电池不可替代的正极材料,具有很高的体积能量密度。钴酸锂的主流 O3 相采用紧密堆积氧原子的 ABCABC(A、B、C 代表紧密堆积平面上的晶格位点)堆积模式。目前,钴酸锂开发的重点是在高电压(相对于 Li+/Li,为 4.55 V)下应用,以获得高比容量(190 mAh g-1)。然而,在高截止电压下循环使用时,O3-LiCoO2 的容量会迅速衰减。失效的原因主要是深度脱铁后向 H1-3/O1 相的不可逆转变以及与电解质的严重界面反应。除 O3 外,已知钴酸锂还会在 ABAC 堆叠的 O2 相中结晶。自发现以来,人们对 O2-LiCoO2 的高压行为知之甚少。本文通过系统比较 O3 和 O2 LiCoO2 在高压下的电化学性能,揭示了在相同微小颗粒形态下,O2-LiCoO2 的稳定性(4.5 V)明显优于 O3-LiCoO2 。结合各种表征技术和多尺度模拟,O2-LiCoO2 优异的高压稳定性归功于其较高的锂扩散率和理想的机械性能。均匀的 Li+ 分布和平衡的内应力负载可能是提高钴酸锂高压性能的关键。本文章由计算机程序翻译,如有差异,请以英文原文为准。
On the Much-Improved High-Voltage Cycling Performance of LiCoO2 by Phase Alteration from O3 to O2 Structure
Lithium cobalt oxide (LiCoO2) is an irreplaceable cathode material for lithium-ion batteries with high volumetric energy density. The prevailing O3 phase LiCoO2 adopts the ABCABC (A, B, and C stand for lattice sites in the close-packed plane) stacking modes of close-packed oxygen atoms. Currently, the focus of LiCoO2 development is application at high voltage (>4.55 V versus Li+/Li) to achieve a high specific capacity (>190 mAh g−1). However, cycled with a high cutoff voltage, O3–LiCoO2 suffers from rapid capacity decay. The causes of failure are mostly attributed to the irreversible transitions to H1-3/O1 phase after deep delithiation and severe interfacial reactions with electrolytes. In addition to O3, LiCoO2 is also known to crystalize in an O2 phase with ABAC stacking. Since its discovery, little is known about the high-voltage behavior of O2–LiCoO2. Herein, through systematic comparison between electrochemical performances of O3 and O2 LiCoO2 at high voltage, the significantly better stability of O2–LiCoO2 (>4.5 V) than that of O3–LiCoO2 in the same micro-sized particle morphology is revealed. Combining various characterization techniques and multiscale simulation, the outstanding high-voltage stability of O2–LiCoO2 is attributed to the high Li diffusivity and ideal mechanical properties. Uniform Li+ distribution and balanced internal stress loading may hold the key to improving the high-voltage performance of LiCoO2.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
14.00
自引率
2.40%
发文量
0
期刊介绍:
Small Science is a premium multidisciplinary open access journal dedicated to publishing impactful research from all areas of nanoscience and nanotechnology. It features interdisciplinary original research and focused review articles on relevant topics. The journal covers design, characterization, mechanism, technology, and application of micro-/nanoscale structures and systems in various fields including physics, chemistry, materials science, engineering, environmental science, life science, biology, and medicine. It welcomes innovative interdisciplinary research and its readership includes professionals from academia and industry in fields such as chemistry, physics, materials science, biology, engineering, and environmental and analytical science. Small Science is indexed and abstracted in CAS, DOAJ, Clarivate Analytics, ProQuest Central, Publicly Available Content Database, Science Database, SCOPUS, and Web of Science.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


