硼烷催化的 2-烷基吡啶†的不对称还原
IF 5.5
1区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
本文首次报道了一种未受保护的 2-烷基吡啶的对映选择性还原方法。以频哪醇硼烷和酰胺为还原剂,通过 1,4-氢化和随后的转移氢化级联过程,合成了大量 2-烷基哌啶类化合物,产量高,对映选择性好。得到的产物可以很容易地转化为天然生物碱。本文章由计算机程序翻译,如有差异,请以英文原文为准。
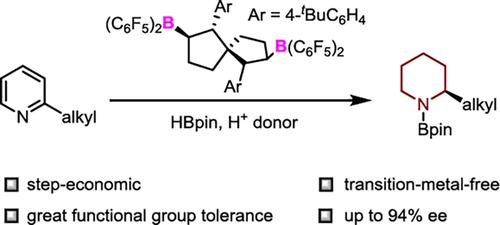
Borane-Catalyzed Asymmetric Reduction of 2-Alkylpyridines†
Herein, a method for the enantioselective reduction of unprotected 2-alkylpyridines is reported for the first time. By using pinacolborane and an amide as reducing agents, a large number of 2-alkylpiperidines were synthesized with high yields and excellent enantioselectivities via a cascade process involving 1,4-hydroboration and subsequent transfer hydrogenation. The resulting products can be easily converted to natural alkaloids.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chinese Journal of Chemistry
化学-化学综合
CiteScore
8.80
自引率
14.80%
发文量
422
审稿时长
1.7 months
期刊介绍:
The Chinese Journal of Chemistry is an international forum for peer-reviewed original research results in all fields of chemistry. Founded in 1983 under the name Acta Chimica Sinica English Edition and renamed in 1990 as Chinese Journal of Chemistry, the journal publishes a stimulating mixture of Accounts, Full Papers, Notes and Communications in English.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


