通过电化学方法生成的手性 α-氨基卡宾化合物中间体进行对映选择性 SN1 型反应
IF 14.7
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
通过碳位中间体进行的电化学反应仍然是以可持续的方式建立分子功能性和复杂性的基本转化过程。在高离子电解质溶液中,对此类过程进行对映选择性控制是一项巨大挑战。在此,我们报告了一种通过烯胺催化阳极生成手性 α-亚氨基碳化中间体的方法。手性碳位中间体可被醇、水和硫醇等多种亲核物截取,并具有很高的立体选择性。关键的 SN1 步骤是通过叔胺介导的质子穿梭来进行的,这有助于在与卡宾化合物反应时进行面选择。本文章由计算机程序翻译,如有差异,请以英文原文为准。
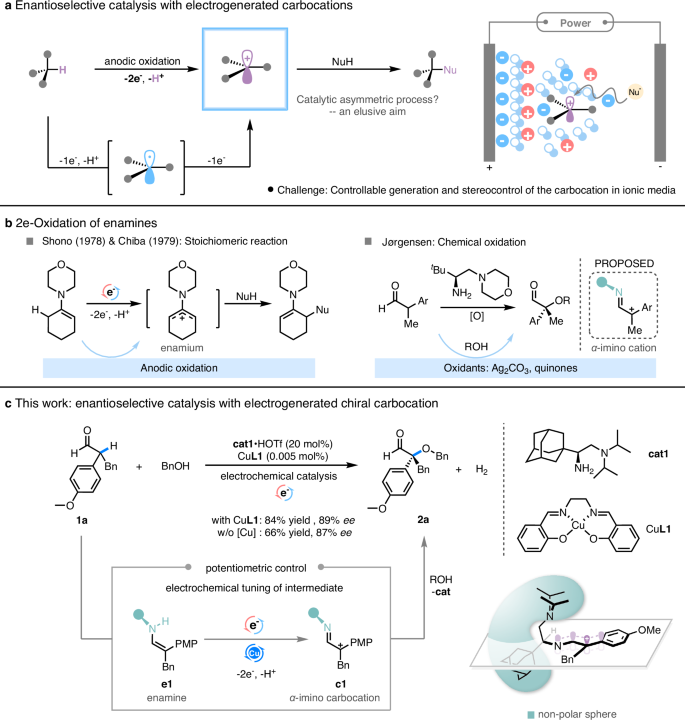
Enantioselective SN1-type reaction via electrochemically generated chiral α-Imino carbocation intermediate
Electrochemical reactions via carbocation intermediates remain fundamental transformations that build up molecular functionality and complexity in a sustainable manner. Enantioselective control of such processes is a great challenge in a highly ionic electrolyte solution. Here, we report an anodic generation of chiral α-imino carbocation intermediates by enamine catalysis. The chiral carbocation intermediates can be intercepted by a variety of nucleophiles such as alcohols, water and thiols with high stereoselectivity. The key SN1 step proceeds via a tertiary amine-mediated proton shuttle that facilitates facial selection in reacting with carbocation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Communications
Biological Science Disciplines-
CiteScore
24.90
自引率
2.40%
发文量
6928
审稿时长
3.7 months
期刊介绍:
Nature Communications, an open-access journal, publishes high-quality research spanning all areas of the natural sciences. Papers featured in the journal showcase significant advances relevant to specialists in each respective field. With a 2-year impact factor of 16.6 (2022) and a median time of 8 days from submission to the first editorial decision, Nature Communications is committed to rapid dissemination of research findings. As a multidisciplinary journal, it welcomes contributions from biological, health, physical, chemical, Earth, social, mathematical, applied, and engineering sciences, aiming to highlight important breakthroughs within each domain.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


