氮化铱使炔烃 C-H 功能化
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
通过亲核芳香取代直接进行炔 C-H 功能化仍然是一项具有挑战性的任务。在此,我们报告了一种由铱腈催化的炔烃 C-H 功能化策略,利用容易获得的芳基叠氮化物作为亲电体,与不同的亲核反应伙伴发生反应。在定制的噁唑啉螯合铱络合物催化下,以 β-萘酚和 β-萘基叠氮化物为起始材料,对映选择性地合成了手性 2-氨基-2′-羟基-1,1′-联萘,这是一类用于不对称转化的构件、配体和催化剂,证明了这一方法的实用性。机理研究和密度泛函理论计算表明,该反应是通过铱亚硝基介导的 C-H 功能化途径进行的。所报道的炔烃 C-H 功能化策略为扩大亲核芳香取代反应的适用性提供了蓝图,对于合成含苯胺的分子尤其有价值。本文章由计算机程序翻译,如有差异,请以英文原文为准。
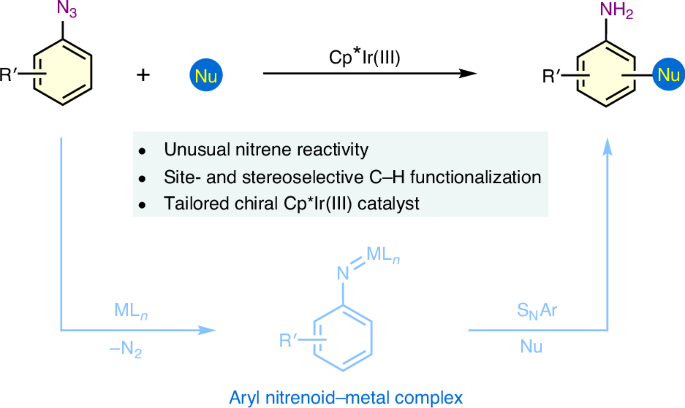
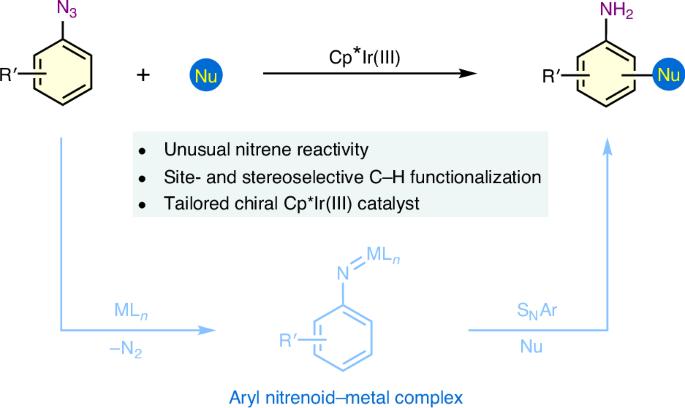
Iridium nitrenoid-enabled arene C−H functionalization
Direct arene C−H functionalization via nucleophilic aromatic substitution remains a challenging task. Here we report an iridium nitrenoid-catalysed arene C−H functionalization strategy, making use of readily available aryl azides as electrophiles to react with different nucleophilic reaction partners. The practicality of this methodology is demonstrated by enantioselective synthesis of chiral 2-amino-2′-hydroxy-1,1′-binaphthyl, a class of building blocks, ligands and catalysts in asymmetric transformation, using β-naphthols and β-naphthyl azides as starting materials under the catalysis of a tailored oxazoline-chelated iridium complex. Mechanistic studies and density functional theory calculations show that the reaction proceeds through an iridium nitrenoid-mediated C−H functionalization pathway. The reported arene C−H functionalization strategy serves as a blueprint to expand the applicability of nucleophilic aromatic substitution reactions and is particularly valuable for the synthesis of aniline-containing molecules. Effective strategies for direct arene C−H functionalization are sought after. Here arene C−H functionalization via iridium nitrenoid-catalysed nucleophilic aromatic substitution allows the coupling of aryl azides with different carbon nucleophiles and is applied to the enantioselective synthesis of NOBINs
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


