必杀丝的自组织及其在细菌分裂环形成中的作用
IF 17.6
1区 物理与天体物理
Q1 PHYSICS, MULTIDISCIPLINARY
引用次数: 0
摘要
细胞中的细丝通常会 "跑步机"。在能量消耗的驱动下,它们的一端增长,而另一端则收缩,从而使丝状物看起来是运动的,即使单个蛋白质保持静止不动。这一过程是细胞骨架细丝的特征,并导致集体细丝自组织。在这里,我们展示了踩踏运动通过溶解错位的细丝来驱动细丝向列有序化。以参与细胞分裂的细菌 FtsZ 蛋白为例,我们展示了这种机制在体外使 FtsZ 细丝排列整齐,并在活的枯草杆菌细胞中驱动分裂环的组织。我们发现,通过局部溶解排序还能使系统快速响应细胞中的化学和几何偏差,从而使我们能够定量解释体内环的形成动态。除了 FtsZ 和其他细胞骨架丝之外,我们的研究还发现了一种通过耗能丝的不断生灭实现自组织的机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
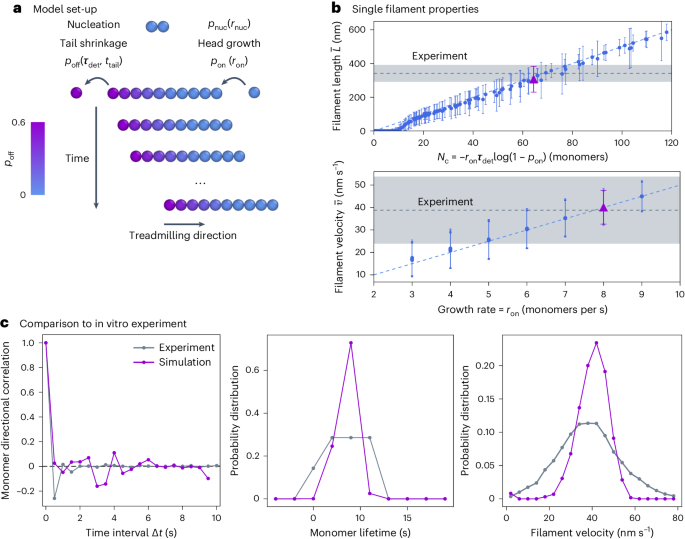
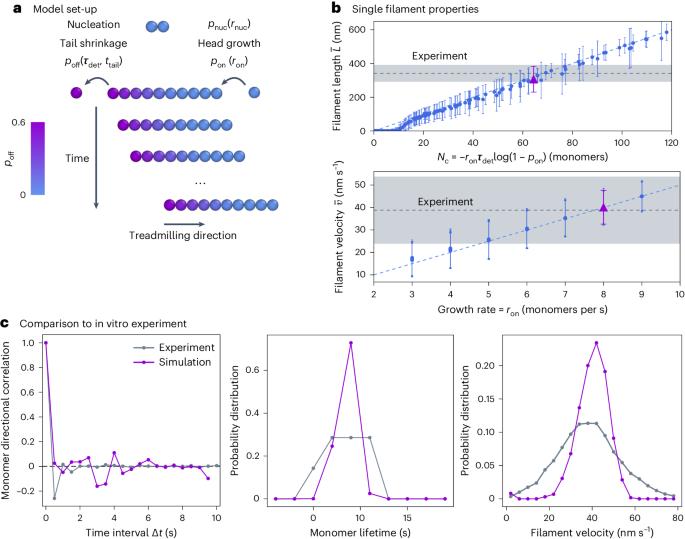
Self-organization of mortal filaments and its role in bacterial division ring formation
Filaments in the cell commonly treadmill. Driven by energy consumption, they grow on one end while shrinking on the other, causing filaments to appear motile even though individual proteins remain static. This process is characteristic of cytoskeletal filaments and leads to collective filament self-organization. Here we show that treadmilling drives filament nematic ordering by dissolving misaligned filaments. Taking the bacterial FtsZ protein involved in cell division as an example, we show that this mechanism aligns FtsZ filaments in vitro and drives the organization of the division ring in living Bacillus subtilis cells. We find that ordering via local dissolution also allows the system to quickly respond to chemical and geometrical biases in the cell, enabling us to quantitatively explain the ring formation dynamics in vivo. Beyond FtsZ and other cytoskeletal filaments, our study identifies a mechanism for self-organization via constant birth and death of energy-consuming filaments. Treadmilling of cytoskeletal filaments is crucial for their functional self-organization. Now the mechanism underpinning this collective organization is shown to be the dissolution of misaligned filaments.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Physics
物理-物理:综合
CiteScore
30.40
自引率
2.00%
发文量
349
审稿时长
4-8 weeks
期刊介绍:
Nature Physics is dedicated to publishing top-tier original research in physics with a fair and rigorous review process. It provides high visibility and access to a broad readership, maintaining high standards in copy editing and production, ensuring rapid publication, and maintaining independence from academic societies and other vested interests.
The journal presents two main research paper formats: Letters and Articles. Alongside primary research, Nature Physics serves as a central source for valuable information within the physics community through Review Articles, News & Views, Research Highlights covering crucial developments across the physics literature, Commentaries, Book Reviews, and Correspondence.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


