金黄色葡萄球菌在慢性感染期间适应利用源自胶原的脯氨酸
IF 20.5
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
摘要
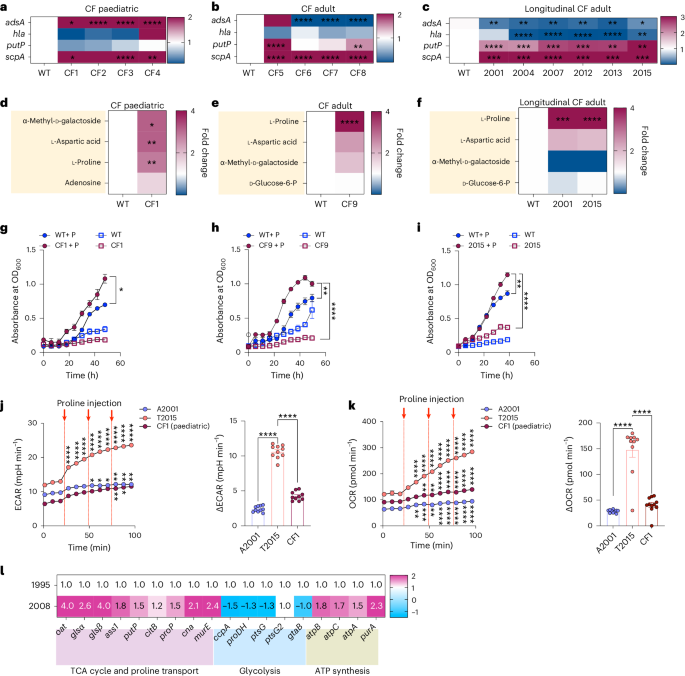
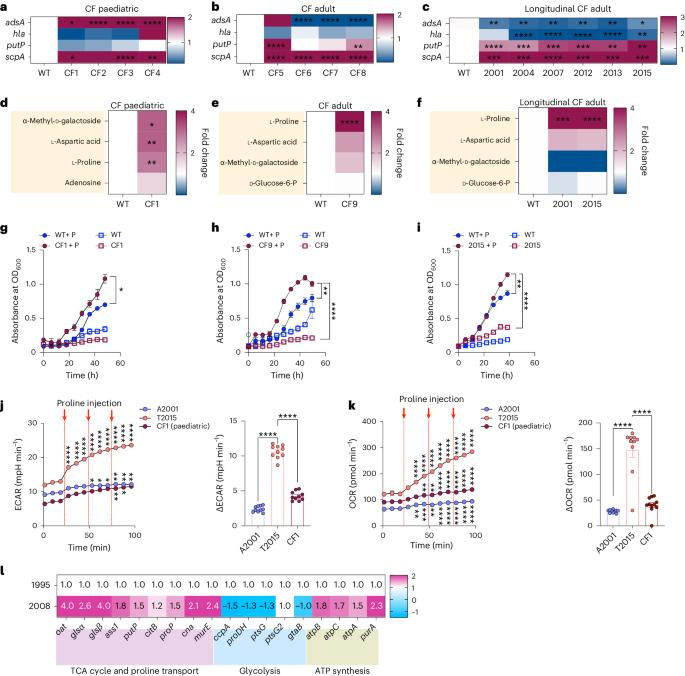
金黄色葡萄球菌是一种肺部病原体,与人类的大量发病和死亡有关。由于针对毒力决定因素的疫苗未能对人类产生保护作用,因此致病机理很可能与其他因素有关。在此,我们分析了初次感染和慢性感染的人类临床分离金黄色葡萄球菌的转录组反应。我们观察到慢性感染分离株的胶原酶和脯氨酸转运体基因表达上调。支气管灌洗液和成纤维细胞感染的代谢组学、生长试验和细菌突变株分析表明,气道成纤维细胞在金黄色葡萄球菌感染期间会产生胶原蛋白。适应宿主的细菌上调胶原酶,从而降解胶原并释放脯氨酸。然后,金黄色葡萄球菌输入脯氨酸,通过三羧酸循环促进氧化代谢。脯氨酸代谢为适应宿主的金黄色葡萄球菌提供了新陈代谢益处,使其能够超越非适应菌株。这些数据表明,以气道修复过程和纤维化为特征的临床环境提供了一个促进金黄色葡萄球菌适应和支持感染的环境。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Staphylococcus aureus adapts to exploit collagen-derived proline during chronic infection
Staphylococcus aureus is a pulmonary pathogen associated with substantial human morbidity and mortality. As vaccines targeting virulence determinants have failed to be protective in humans, other factors are likely involved in pathogenesis. Here we analysed transcriptomic responses of human clinical isolates of S. aureus from initial and chronic infections. We observed upregulated collagenase and proline transporter gene expression in chronic infection isolates. Metabolomics of bronchiolar lavage fluid and fibroblast infection, growth assays and analysis of bacterial mutant strains showed that airway fibroblasts produce collagen during S. aureus infection. Host-adapted bacteria upregulate collagenase, which degrades collagen and releases proline. S. aureus then imports proline, which fuels oxidative metabolism via the tricarboxylic acid cycle. Proline metabolism provides host-adapted S. aureus with a metabolic benefit enabling out-competition of non-adapted strains. These data suggest that clinical settings characterized by airway repair processes and fibrosis provide a milieu that promotes S. aureus adaptation and supports infection. Staphylococcus aureus upregulates collagenase and proline transporters to release collagen-derived proline and exploit fibroblasts as a nutrient source during chronic infection.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Microbiology
Immunology and Microbiology-Microbiology
CiteScore
44.40
自引率
1.10%
发文量
226
期刊介绍:
Nature Microbiology aims to cover a comprehensive range of topics related to microorganisms. This includes:
Evolution: The journal is interested in exploring the evolutionary aspects of microorganisms. This may include research on their genetic diversity, adaptation, and speciation over time.
Physiology and cell biology: Nature Microbiology seeks to understand the functions and characteristics of microorganisms at the cellular and physiological levels. This may involve studying their metabolism, growth patterns, and cellular processes.
Interactions: The journal focuses on the interactions microorganisms have with each other, as well as their interactions with hosts or the environment. This encompasses investigations into microbial communities, symbiotic relationships, and microbial responses to different environments.
Societal significance: Nature Microbiology recognizes the societal impact of microorganisms and welcomes studies that explore their practical applications. This may include research on microbial diseases, biotechnology, or environmental remediation.
In summary, Nature Microbiology is interested in research related to the evolution, physiology and cell biology of microorganisms, their interactions, and their societal relevance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


