用穿流电极取代流动电极,将铜-tmpa 氧还原的极限电流提高 20 倍
IF 7.3
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
过氧化氢(H2O2)是一种绿色氧化剂,在化学工业、水处理和燃料电池的许多(新兴)应用中都有广泛的应用。O2 在水中的低溶解度会导致严重的传质限制,并在工业相关的电流密度下丧失 H2O2 的选择性,从而使实际规模的电化学 H2O2 合成系统的开发复杂化。我们测试了电化学流动池中的并流、直流配置和悬浮电极,以研究电极配置和流动条件对传质和 H2O2 产量的影响。我们使用 Cu-tmpa(tmpa = 三(2-吡啶基甲基)胺)作为均相铜基催化剂,在 pH 值中性的磷酸盐缓冲液中催化 1 小时,监测 H2O2 的产生,并通过 CV 扫描估算极限电流密度。由于增加了表面积和泡沫结构,改善了传质,因此与逐流结构相比,我们在直通结构中获得了最高的 H2O2 产量和 15-20 倍的几何极限电流密度。悬浮电极中的活性炭(AC)材料具有更大的表面积,但会分解产生的所有 H2O2,因此不适合 H2O2 合成。虽然在微观尺度的流过式系统中,传质限制似乎得到了缓解,但高 O2 消耗和 H2O2 产生给维持最初达到的电流密度和法拉第效率(FE)带来了挑战。散装电解液中 O2 和 H2O2 浓度之间的比率不断降低,这可能会给使用较长电极的大型系统带来挑战。调整反应器的设计和运行条件对于最大限度地提高法拉第效率和电流密度至关重要。本文章由计算机程序翻译,如有差异,请以英文原文为准。
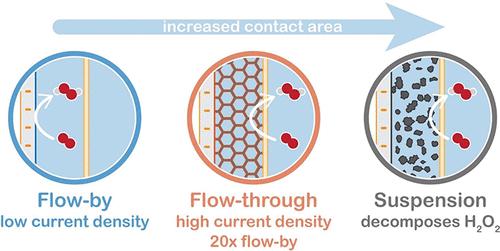
20-Fold Increased Limiting Currents in Oxygen Reduction with Cu-tmpa by Replacing Flow-By with Flow-Through Electrodes
Electrochemical oxygen reduction is a promising and sustainable alternative to the current industrial production method for hydrogen peroxide (H2O2), which is a green oxidant in many (emerging) applications in the chemical industry, water treatment, and fuel cells. Low solubility of O2 in water causes severe mass transfer limitations and loss of H2O2 selectivity at industrially relevant current densities, complicating the development of practical-scale electrochemical H2O2 synthesis systems. We tested a flow-by and flow-through configuration and suspension electrodes in an electrochemical flow cell to investigate the influence of electrode configuration and flow conditions on mass transfer and H2O2 production. We monitored the H2O2 production using Cu-tmpa (tmpa = tris(2-pyridylmethyl)amine) as a homogeneous copper-based catalyst in a pH-neutral phosphate buffer during 1 h of catalysis and estimated the limiting current density from CV scans. We achieve the highest H2O2 production and a 15–20 times higher geometrical limiting current density in the flow-through configuration compared to the flow-by configuration due to the increased surface area and foam structure that improved mass transfer. The activated carbon (AC) material in suspension electrodes, which have an even larger surface area, decomposes all produced H2O2 and proves unsuitable for H2O2 synthesis. Although the mass transfer limitations seem to be alleviated on the microscale in the flow-through system, the high O2 consumption and H2O2 production cause challenges in maintaining the initially reached current density and Faradaic efficiency (FE). The decreasing ratio between the concentrations of the O2 and H2O2 in the bulk electrolyte will likely pose a challenge when proceeding to larger systems with longer electrodes. Tuning the reactor design and operating conditions will be essential in maximizing the FE and current density.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


