铜催化氧化分子内环化合成 2-羟基-吲哚啉-3-酮
IF 4.4
2区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
摘要
由于许多天然产物和生物活性分子中经常出现吲哚核,取代的 2-羟基-吲哚啉-3-酮的合成引起了广泛关注。本文开发了一种通过铜催化 N-(2-乙酰苯基)吡啶酰胺的氧化分子内环化直接获得 2-羟基-吲哚啉-3-酮的方法。该方法具有良好的官能团耐受性和原子经济性,并避免了底物的预官能化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
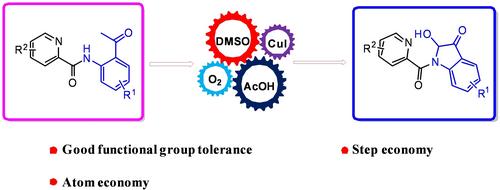
Copper‐Catalyzed Oxidative Intramolecular Cyclization for the Synthesis of 2‐Hydroxy‐Indolin‐3‐Ones
The synthesis of substituted 2‐hydroxy‐indolin‐3‐ones has attracted considerable attention due to the frequent presence of the indole nucleus in numerous natural products and biologically active molecules. Herein, a direct access to 2‐hydroxy‐indolin‐3‐ones through copper‐catalyzed oxidative intramolecular cyclization of N‐(2‐acetylphenyl)picolinamide has been developed. This method exhibits good functional group tolerance, atom‐economy and avoids the pre‐functionalization of substrates.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Advanced Synthesis & Catalysis
化学-应用化学
CiteScore
9.40
自引率
7.40%
发文量
447
审稿时长
1.8 months
期刊介绍:
Advanced Synthesis & Catalysis (ASC) is the leading primary journal in organic, organometallic, and applied chemistry.
The high impact of ASC can be attributed to the unique focus of the journal, which publishes exciting new results from academic and industrial labs on efficient, practical, and environmentally friendly organic synthesis. While homogeneous, heterogeneous, organic, and enzyme catalysis are key technologies to achieve green synthesis, significant contributions to the same goal by synthesis design, reaction techniques, flow chemistry, and continuous processing, multiphase catalysis, green solvents, catalyst immobilization, and recycling, separation science, and process development are also featured in ASC. The Aims and Scope can be found in the Notice to Authors or on the first page of the table of contents in every issue.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


