光脱附光电子能谱显示了酚类硝酸盐复合物中的同分异构质子耦合电子转移反应。
IF 5.9
2区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
酚类化合物的氧化是各种生物过程中普遍存在的最重要反应之一,通常与质子转移(PTs)明确耦合。对这些质子耦合电子转移(PCET)反应的定量描述和分子水平的理解一直具有挑战性。这项研究报告了在氢键酚(ArOH)硝酸酯(NO3-)复合物的光脱离(PD)光电子能谱(PES)中直接观察到的 PCET,其中更慢的上升沿提供了证明 PCET 的光谱特征。电子结构计算揭示了 PCET 过程具有异构体特异性,只发生在 HOMO 定位于 ArOH 的异构体中,导致电离酚引发的电荷分离瞬态 ArOH-+-NO3-,同时促进从 ArOH-+ 到 NO3- 的 PT。重要的是,这项研究表明气相 PD-PES 是一种通用的方法,可以利用明确的结构和结合信息识别 PCET 反应。本文章由计算机程序翻译,如有差异,请以英文原文为准。
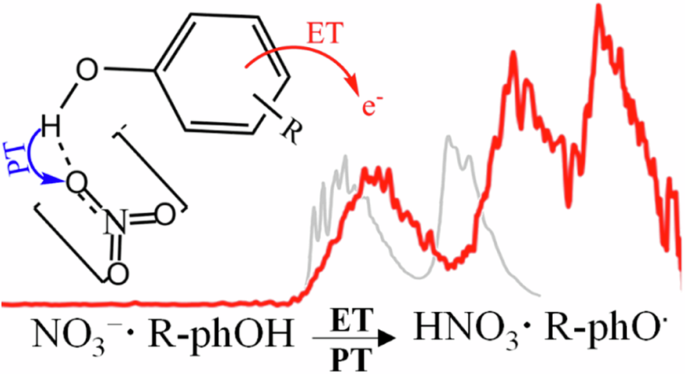
Photodetachment photoelectron spectroscopy shows isomer-specific proton-coupled electron transfer reactions in phenolic nitrate complexes
The oxidation of phenolic compounds is one of the most important reactions prevalent in various biological processes, often explicitly coupled with proton transfers (PTs). Quantitative descriptions and molecular-level understanding of these proton-coupled electron transfer (PCET) reactions have been challenging. This work reports a direct observation of PCET in photodetachment (PD) photoelectron spectroscopy (PES) of hydrogen-bonded phenolic (ArOH) nitrate (NO3−) complexes, in which a much slower rising edge provides a spectroscopic signature to evidence PCET. Electronic structure calculations unveil the PCET processes to be isomer-specific, occurred only in those with their HOMOs localized on ArOH, leading to charge-separated transient states ArOH•+·NO3− triggered by ionizing phenols while simultaneously promoting PT from ArOH•+ to NO3−. Importantly, this study showcases that gas-phase PD-PES is a generic means enabling to identify PCET reactions with explicit structural and binding information. The oxidation of phenolic compounds is one of the most important reactions prevalent in various biological processes, but quantitative descriptions and molecular-level understanding of these proton-coupled electron transfer (PCET) reactions have been challenging. Here, the authors use photodetachment photoelectron spectroscopy to directly observe PCET in hydrogen-bonded phenolic nitrate complexes, in which a much slower rising edge provides a spectroscopic signature to evidence PCET
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Communications Chemistry
Chemistry-General Chemistry
CiteScore
7.70
自引率
1.70%
发文量
146
审稿时长
13 weeks
期刊介绍:
Communications Chemistry is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the chemical sciences. Research papers published by the journal represent significant advances bringing new chemical insight to a specialized area of research. We also aim to provide a community forum for issues of importance to all chemists, regardless of sub-discipline.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


