光诱导碘催化的烯丙酮脱烷基合成反应
IF 4.4
2区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
摘要
通过 C-N 键裂解,描述了光介导的碘催化合成烯酮。在标准条件下,除取代的炔酮外,各种取代的无环叔胺和环叔胺也能发生反应。耦合加合物的部分转化证明了这一方案的潜在用途。此外,还进行了初步的机理研究。本文章由计算机程序翻译,如有差异,请以英文原文为准。
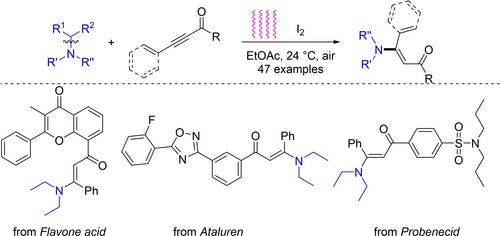
Light‐Induced Iodine‐Catalyzed Dealkylative Synthesis of Enaminones
Light mediated iodine‐catalyzed synthesis of enaminones through C−N bond cleavage has been described. In addition to substituted ynones, diverse substituted acyclic tertiary amines, and cyclic tertiary amines were also reactive under standard conditions. Selected transformations of the coupling adduct demonstrated the latent utility of this protocol. Preliminary mechanistic studies were also conducted.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Advanced Synthesis & Catalysis
化学-应用化学
CiteScore
9.40
自引率
7.40%
发文量
447
审稿时长
1.8 months
期刊介绍:
Advanced Synthesis & Catalysis (ASC) is the leading primary journal in organic, organometallic, and applied chemistry.
The high impact of ASC can be attributed to the unique focus of the journal, which publishes exciting new results from academic and industrial labs on efficient, practical, and environmentally friendly organic synthesis. While homogeneous, heterogeneous, organic, and enzyme catalysis are key technologies to achieve green synthesis, significant contributions to the same goal by synthesis design, reaction techniques, flow chemistry, and continuous processing, multiphase catalysis, green solvents, catalyst immobilization, and recycling, separation science, and process development are also featured in ASC. The Aims and Scope can be found in the Notice to Authors or on the first page of the table of contents in every issue.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


