一种显示氢键融合的双功能电解质添加剂可实现高度可逆的锌离子水电池。
IF 5.9
2区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
近年来,水性锌离子电池(AZIBs)因其安全性高、成本低、容量大和环保等显著优势,在储能领域备受关注。然而,氢进化和锌(Zn)枝晶等副反应会严重影响其库仑效率(CE)和使用寿命。有效解决这些问题已成为该领域的研究重点。在我们的研究中,二甲基亚砜(DMSO)和纳米金刚石(NDs)被用来优化 AZIB 的电解质。二甲基亚砜和纳米金刚石的氢键融合调节了锌的沉积行为,有效抑制了锌枝晶的生长、氢演化和腐蚀。使用 NDs-DMSO-ZS 的 Zn | |Zn 对称电池在 1 mA cm-2 下的循环稳定性超过 1500 小时,而 Zn//Cu 不对称电池在 2 mA cm-2 下的 CE 高达 99.8%。这项研究不仅展示了电解质优化在提高 AZIBs 性能方面的应用前景,也为先进 AZIBs 技术中电解质技术的进步提供了参考。本文章由计算机程序翻译,如有差异,请以英文原文为准。
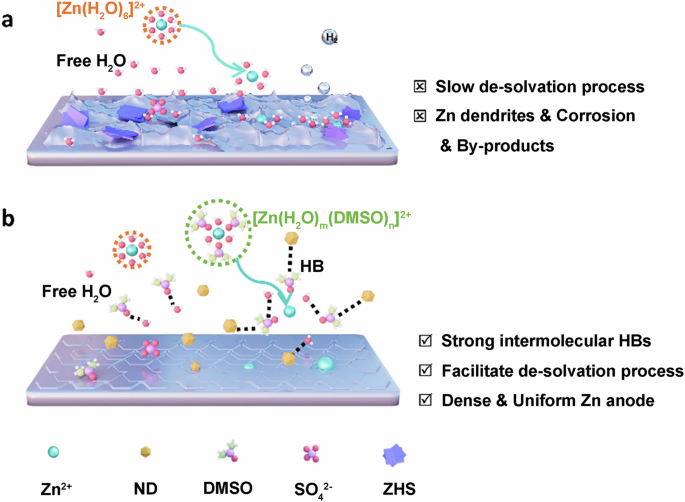
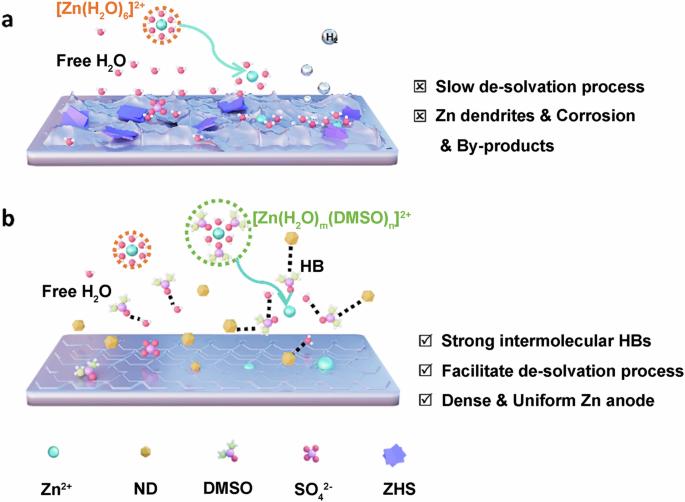
A dual-functional electrolyte additive displaying hydrogen bond fusion enables highly reversible aqueous zinc ion batteries
In recent years, aqueous zinc-ion batteries (AZIBs) have attracted significant attention in energy storage due to their notable advantages, including high safety, low cost, high capacity, and environmental friendliness. However, side reactions like hydrogen evolution and zinc (Zn) dendrites can significantly impact their Coulombic efficiency (CE) and lifespan. Effectively addressing these issues has become a focus of research in this field. In our study, dimethyl sulfoxide (DMSO) and nanodiamonds (NDs) were used to optimize the electrolyte of AZIBs. Benefiting from the hydrogen bond fusion of DMSO and NDs, which regulates the Zn deposition behavior, effectively inhibiting the growth of Zn dendrites, hydrogen evolution, and corrosion. The Zn | |Zn symmetric cells using NDs-DMSO-ZS demonstrate exceptional cycling stability for over 1500 h at 1 mA cm−2, while the Zn//Cu asymmetric cells achieve up to 99.8% CE at 2 mA cm−2. This study not only shows the application prospects of electrolyte optimization in enhancing AZIBs performance, but also provides a reference for the advancement of electrolyte technology in advanced AZIBs technology. Aqueous zinc ion batteries represent promising next-generation energy storage systems, but unwanted side reactions such as hydrogen evolution and zinc dendrite formation can significantly impact their Coulombic efficiency and lifespan. Here, dimethyl sulfoxide and nanodiamond additives are introduced to a ZnSO4 electrolyte, effectively inhibiting side reactions and dendrite formation through hydrogen bond fusion, and enabling exceptional cycling stability for over 1500 h at 1 mA cm−2.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Communications Chemistry
Chemistry-General Chemistry
CiteScore
7.70
自引率
1.70%
发文量
146
审稿时长
13 weeks
期刊介绍:
Communications Chemistry is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the chemical sciences. Research papers published by the journal represent significant advances bringing new chemical insight to a specialized area of research. We also aim to provide a community forum for issues of importance to all chemists, regardless of sub-discipline.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


