参与蒽环类药物 1-羟基化的双组分单加氧酶的作用机制
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
蒽环类化合物是微生物天然产物,具有重要的抗增殖生物活性,被广泛用于抗癌化疗。包括诺加霉素和柯西霉素在内的几种蒽环类化合物含有一个 1-羟基,该羟基由一个非典型的双组分单加氧酶系统安装。在这里,我们阐明了 1-羟基化的结构和机理基础。我们展示了 NADPH 依赖性还原酶 SwaQ2 与多柔比星复合物的晶体结构,该结构表明反应是由醌还原引发的。还原后的蒽环类配体可能会与分子氧发生反应,形成与黄素化学类似的过氧化物中间体。参与诺加霉素生物合成的类多酮环化酶 SnoaL2 与底物和产物的复合物结构揭示了一种新的催化四分体,它用于稳定还原反应中间体,将反应引向 1-羟基化。此外,我们还报告了几种未知蒽环类 1-羟化酶的特征,它们显示出不同的底物特征。通过研究柯西诺司他汀途径中类似多酮环化酶的 KstA15 的结构,基于结构的蛋白质工程得以扩展该酶的底物特异性,使其包括糖基化的蒽环类化合物。我们的工作让人们深入了解了还原酶-羟化酶双组分系统如何在几种蒽环类药物途径中避开有机辅助因子或金属离子来催化单氧化反应。本文章由计算机程序翻译,如有差异,请以英文原文为准。
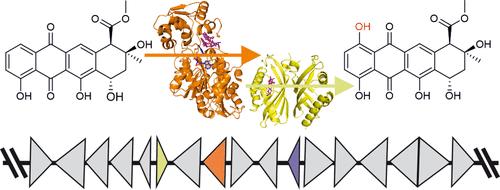
Mechanism of Two-Component Mono-Oxygenases Involved in Anthracycline 1-Hydroxylation
Anthracyclines are microbial natural products with important antiproliferative bioactivities that are widely used in anticancer chemotherapy. Several anthracyclines, including nogalamycin and kosinostatin, contain a 1-hydroxyl group, which is installed by an atypical two-component mono-oxygenase system. Here, we clarify the structural and mechanistic basis for 1-hydroxylation. We present the crystal structure of the NADPH-dependent reductase SwaQ2 in complex with doxorubicin, which indicates that the reaction is initiated by quinone reduction. The reduced anthracycline ligand may react with molecular oxygen, leading to the formation of a peroxide intermediate similar to flavin chemistry. The structures of the polyketide cyclase-like SnoaL2, involved in nogalamycin biosynthesis, in complex with substrate and product reveal a novel catalytic tetrad, which is used to stabilize a reduced reaction intermediate to direct the reaction toward 1-hydroxylation. Furthermore, we report the characterization of several unknown anthracycline 1-hydroxylases, which display varied substrate profiles. The structure of polyketide cyclase-like KstA15 from the kosinostatin pathway enabled structure-based protein engineering to expand the substrate specificity of the enzyme to include glycosylated anthracyclines. Our work provides insight into how reductase-hydroxylase two-component systems circumvent the need for organic cofactors or metal ions to catalyze monooxygenations in several anthracycline pathways.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


