人类前导链 DNA 聚合酶 Pol ε 进行子链合成和校对的结构基础
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
在染色体复制过程中,新生前导链由 DNA 聚合酶ε(Pol ε)合成,它与滑动钳加工因子增殖细胞核抗原(PCNA)结合形成一个加工全酶。Pol ε依靠核苷酸选择性及其校对能力来检测和切除错误结合的核苷酸,从而实现高保真的DNA合成。在这里,我们展示了人Pol ε与PCNA、DNA和输入核苷酸复合物的冷冻电子显微镜(cryo-EM)结构,揭示了Pol ε如何通过其PCNA-interacting肽盒与PCNA结合,以及其催化结构域的其他独特特征。此外,通过解决 Pol ε 在含错配 DNA 上的一系列低温电子显微镜结构,我们阐明了 Pol ε 如何感知和编辑误入的核苷酸。我们的结构描述了聚合酶和外切酶活性之间分子内切换机制的步骤,为 B-家族复制聚合酶的校对机制提供了基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
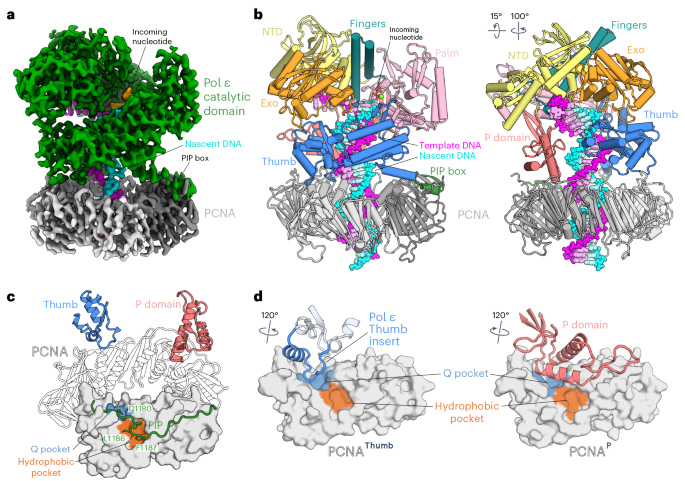

Structural basis for processive daughter-strand synthesis and proofreading by the human leading-strand DNA polymerase Pol ε
During chromosome replication, the nascent leading strand is synthesized by DNA polymerase epsilon (Pol ε), which associates with the sliding clamp processivity factor proliferating cell nuclear antigen (PCNA) to form a processive holoenzyme. For high-fidelity DNA synthesis, Pol ε relies on nucleotide selectivity and its proofreading ability to detect and excise a misincorporated nucleotide. Here, we present cryo-electron microscopy (cryo-EM) structures of human Pol ε in complex with PCNA, DNA and an incoming nucleotide, revealing how Pol ε associates with PCNA through its PCNA-interacting peptide box and additional unique features of its catalytic domain. Furthermore, by solving a series of cryo-EM structures of Pol ε at a mismatch-containing DNA, we elucidate how Pol ε senses and edits a misincorporated nucleotide. Our structures delineate steps along an intramolecular switching mechanism between polymerase and exonuclease activities, providing the basis for a proofreading mechanism in B-family replicative polymerases. Using cryo-electron microscopy, the authors deepen our mechanistic understanding of nascent leading-strand synthesis during human DNA replication and provide the basis for a proofreading mechanism in B-family replicative polymerases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


