Daiana A. De Souza, Max Feken, Hudson V. V. Tomé, Daniel R. Schmehl
下载PDF
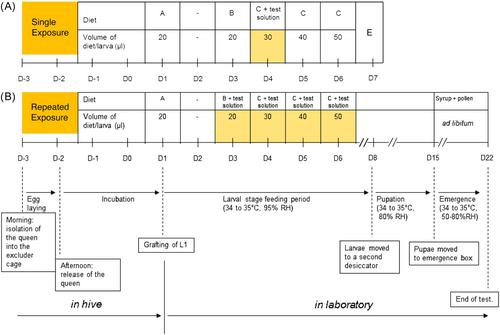
{"title":"蜜蜂幼虫毒性研究设计:当前研究方案和终点的适用性,作为预测农药对传粉昆虫危害的指标。","authors":"Daiana A. De Souza, Max Feken, Hudson V. V. Tomé, Daniel R. Schmehl","doi":"10.1002/ieam.4982","DOIUrl":null,"url":null,"abstract":"<p>The assessment of pesticide risks to bees in North America currently relies in part on Tier 1 honey bee laboratory toxicity studies to support the registration and registration review processes for crop protection chemicals. For immature stages, the studies follow two standardized test designs recommended by the Organization for Economic Cooperation (OECD), evaluating acute (seven-day single-dose, TG OECD 237) and chronic (22-day repeated-dose, GD OECD 239) toxicity in bee larvae. In this article, we aim to evaluate the current approach for generating and interpreting honey bee larval toxicity data, enhancing pesticide risk assessment for pollinators. First, by considering that the repeated-dose larval study covers all stages of honey bee brood development up to adult emergence, we compared endpoints (larval LD/ED50 and LC/EC50 values) from seven-day acute exposure studies with the 22-day chronic exposure studies. Our goal was to identify the study design offering greater sensitivity in assessing pesticide toxicity to immature bees. Our second objective involved analyzing available weight data from emerged adults and comparing it to survival endpoints (e.g., NOEL and LD50) to determine if the weight after adult emergence would accurately represent a sensitive indicator of pesticide effects on developing honey bees. Our analysis determined that the use of a single 22-day chronic exposure study adequately covers all immature stages and that the toxicity values based on cumulative dose are more accurate and representative measures of exposure for immature bees than using endpoints based on estimated daily doses. Furthermore, our analysis suggests that measuring the weight of emerged adults was a more sensitive indicator than mortality of treatment-related effects in 22% of the compounds included in our analysis. Here we also discuss the importance of standardized protocols for proper collection of weight after emergence and the need for further discussion on the relevance of this parameter at risk assessment scheme. <i>Integr Environ Assess Manag</i> 2024;20:2283–2293. © 2024 Pollinator Research Task Force. <i>Integrated Environmental Assessment and Management</i> published by Wiley Periodicals LLC on behalf of Society of Environmental Toxicology & Chemistry (SETAC).</p>","PeriodicalId":13557,"journal":{"name":"Integrated Environmental Assessment and Management","volume":"20 6","pages":"2283-2293"},"PeriodicalIF":3.0000,"publicationDate":"2024-08-07","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/ieam.4982","citationCount":"0","resultStr":"{\"title\":\"Honey bee larval toxicity study designs: Applicability of the current study protocols and endpoints as a predictor of pesticide hazard for pollinators\",\"authors\":\"Daiana A. De Souza, Max Feken, Hudson V. V. Tomé, Daniel R. Schmehl\",\"doi\":\"10.1002/ieam.4982\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"<p>The assessment of pesticide risks to bees in North America currently relies in part on Tier 1 honey bee laboratory toxicity studies to support the registration and registration review processes for crop protection chemicals. For immature stages, the studies follow two standardized test designs recommended by the Organization for Economic Cooperation (OECD), evaluating acute (seven-day single-dose, TG OECD 237) and chronic (22-day repeated-dose, GD OECD 239) toxicity in bee larvae. In this article, we aim to evaluate the current approach for generating and interpreting honey bee larval toxicity data, enhancing pesticide risk assessment for pollinators. First, by considering that the repeated-dose larval study covers all stages of honey bee brood development up to adult emergence, we compared endpoints (larval LD/ED50 and LC/EC50 values) from seven-day acute exposure studies with the 22-day chronic exposure studies. Our goal was to identify the study design offering greater sensitivity in assessing pesticide toxicity to immature bees. Our second objective involved analyzing available weight data from emerged adults and comparing it to survival endpoints (e.g., NOEL and LD50) to determine if the weight after adult emergence would accurately represent a sensitive indicator of pesticide effects on developing honey bees. Our analysis determined that the use of a single 22-day chronic exposure study adequately covers all immature stages and that the toxicity values based on cumulative dose are more accurate and representative measures of exposure for immature bees than using endpoints based on estimated daily doses. Furthermore, our analysis suggests that measuring the weight of emerged adults was a more sensitive indicator than mortality of treatment-related effects in 22% of the compounds included in our analysis. Here we also discuss the importance of standardized protocols for proper collection of weight after emergence and the need for further discussion on the relevance of this parameter at risk assessment scheme. <i>Integr Environ Assess Manag</i> 2024;20:2283–2293. © 2024 Pollinator Research Task Force. <i>Integrated Environmental Assessment and Management</i> published by Wiley Periodicals LLC on behalf of Society of Environmental Toxicology & Chemistry (SETAC).</p>\",\"PeriodicalId\":13557,\"journal\":{\"name\":\"Integrated Environmental Assessment and Management\",\"volume\":\"20 6\",\"pages\":\"2283-2293\"},\"PeriodicalIF\":3.0000,\"publicationDate\":\"2024-08-07\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"https://onlinelibrary.wiley.com/doi/epdf/10.1002/ieam.4982\",\"citationCount\":\"0\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"Integrated Environmental Assessment and Management\",\"FirstCategoryId\":\"93\",\"ListUrlMain\":\"https://onlinelibrary.wiley.com/doi/10.1002/ieam.4982\",\"RegionNum\":4,\"RegionCategory\":\"环境科学与生态学\",\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"Q2\",\"JCRName\":\"ENVIRONMENTAL SCIENCES\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"Integrated Environmental Assessment and Management","FirstCategoryId":"93","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/ieam.4982","RegionNum":4,"RegionCategory":"环境科学与生态学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q2","JCRName":"ENVIRONMENTAL SCIENCES","Score":null,"Total":0}
引用次数: 0
引用
批量引用

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


