量子约束诱导的金属镍氢氧化电催化剂的抗电氧化作用
IF 49.7
1区 材料科学
Q1 ENERGY & FUELS
引用次数: 0
摘要
阴离子交换膜燃料电池(AEMFC)是一种极具吸引力和成本效益的能源转换技术,因为它可以使用地球上丰富且低成本的非贵金属催化剂。然而,AEMFC 中用于催化氢氧化反应的非贵金属容易发生自氧化,导致不可逆转的失效。在这里,我们展示了一种量子井状催化结构(QWCS),它是通过将镍纳米粒子原子限制在掺碳的氧化钼/氧化钼异质结(C-MoOx/MoOx)中而构建的,可以选择性地转移氢氧化反应中的外部电子,同时自身仍保持金属性。镍纳米粒子的电子获得了 QWCS 提供的 1.11 eV 的势垒,从而使镍相对于可逆氢电极(VRHE)的稳定性高达 1.2 V。QWCS 催化的 AEMFC 实现了 486 mW mgNi-1 的高功率密度,并在关机-启动循环期间经受住了氢饥饿操作,而没有 QWCS 的对应 AEMFC 在一个循环中就失效了。本文章由计算机程序翻译,如有差异,请以英文原文为准。
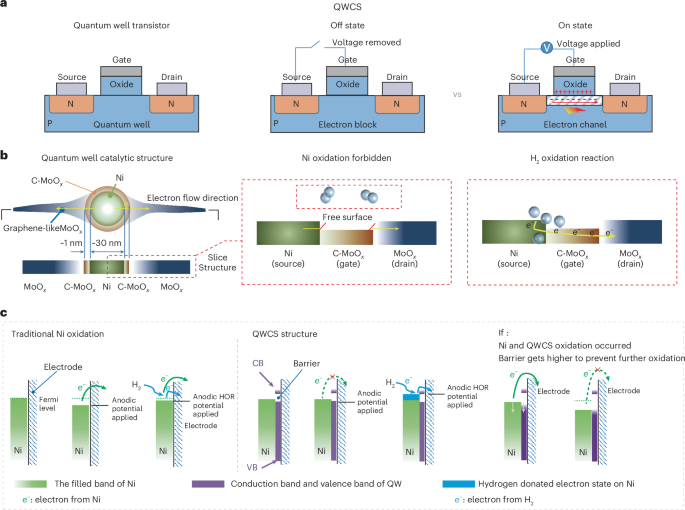
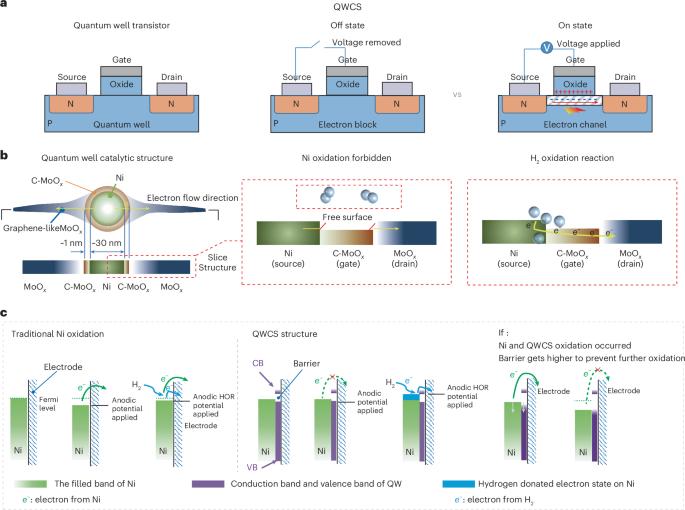
Quantum confinement-induced anti-electrooxidation of metallic nickel electrocatalysts for hydrogen oxidation
The anion-exchange-membrane fuel cell (AEMFC) is an attractive and cost-effective energy-conversion technology because it can use Earth-abundant and low-cost non-precious metal catalysts. However, non-precious metals used in AEMFCs to catalyse the hydrogen oxidation reaction are prone to self-oxidation, resulting in irreversible failure. Here we show a quantum well-like catalytic structure (QWCS), constructed by atomically confining Ni nanoparticles within a carbon-doped-MoOx/MoOx heterojunction (C-MoOx/MoOx) that can selectively transfer external electrons from the hydrogen oxidation reaction while remaining itself metallic. Electrons of Ni nanoparticles gain a barrier of 1.11 eV provided by the QWCS leading to Ni stability up to 1.2 V versus the reversible hydrogen electrode (VRHE) whereas electrons released from the hydrogen oxidation reaction easily cross the barrier by a gating operation of QWCS upon hydrogen adsorption. The QWCS-catalysed AEMFC achieved a high-power density of 486 mW mgNi−1 and withstood hydrogen starvation operations during shutdown–start cycles, whereas a counterpart AEMFC without QWCS failed in a single cycle. Non-precious metals used at the anode of anion-exchange-membrane fuel cells to catalyse hydrogen oxidation are prone to self-oxidation. Here Zhou and colleagues report that a quantum well-like catalytic structure containing Ni nanoparticles within a C-doped MoOx/MoOx heterojunction can mitigate such degradation by a gating operation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Energy
Energy-Energy Engineering and Power Technology
CiteScore
75.10
自引率
1.10%
发文量
193
期刊介绍:
Nature Energy is a monthly, online-only journal committed to showcasing the most impactful research on energy, covering everything from its generation and distribution to the societal implications of energy technologies and policies.
With a focus on exploring all facets of the ongoing energy discourse, Nature Energy delves into topics such as energy generation, storage, distribution, management, and the societal impacts of energy technologies and policies. Emphasizing studies that push the boundaries of knowledge and contribute to the development of next-generation solutions, the journal serves as a platform for the exchange of ideas among stakeholders at the forefront of the energy sector.
Maintaining the hallmark standards of the Nature brand, Nature Energy boasts a dedicated team of professional editors, a rigorous peer-review process, meticulous copy-editing and production, rapid publication times, and editorial independence.
In addition to original research articles, Nature Energy also publishes a range of content types, including Comments, Perspectives, Reviews, News & Views, Features, and Correspondence, covering a diverse array of disciplines relevant to the field of energy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


