一氧化碳促成协同羰基化和(杂)芳基迁移
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
一氧化碳(CO)是一种用途广泛的 C1 原料,其活化和转化一直备受关注。传统上,研究人员一直专注于开发催化系统,以活化一氧化碳,然后用亲核物淬灭生成的酰基中间体,完成羰基转化。在这里,我们非经典地揭示了一种可见光诱导、羰基化触发的自由基中继重排反应,其中 CO 插入步骤是官能团迁移的关键因素。选择性地将羰基插入新生成的碳自由基为(杂)芳基迁移提供了桥梁,而基团迁移的正反馈也使碳自由基能够更有效地捕获 CO。在温和的条件下成功合成了一系列含有氟烷基和杂环的 1,4-二羰基化合物,并将产物转化为有价值的杂芳香族双芳基化合物,显示了这一平台的合成潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
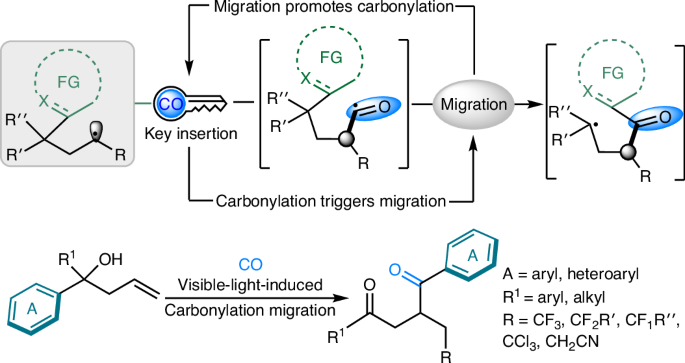
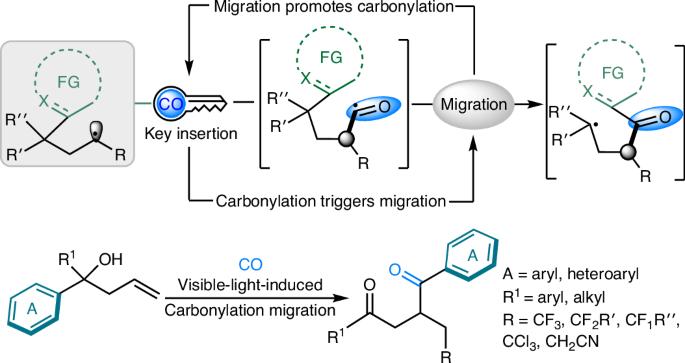
Carbon monoxide enabling synergistic carbonylation and (hetero)aryl migration
The activation and transformation of carbon monoxide (CO), a versatile C1 feedstock, continues to attract substantial attention. Traditionally, researchers have focused on the development of catalytic systems to activate CO and then quench the generated acyl intermediate with nucleophiles to complete the carbonylative transformations. Here, non-classically, we unveil a visible-light-induced, carbonylation-triggered radical relay rearrangement reaction, in which the CO insertion step is a key element for functional group migration. The selective insertion of a carbonyl group into the newly generated carbon radical provides a bridge for (hetero)aryl group migration, and the positive feedback of group migration also enables the carbon radical to capture CO more efficiently. A series of 1,4-dicarbonyl compounds containing fluoroalkyl and heterocycles are synthesized successfully under mild conditions, and the conversion of the products to valuable heteroaromatic biaryls indicates the synthetic potential of this platform. The activation and transformation of carbon monoxide (CO) continues to attract much attention. Now, the authors report a visible-light-induced, carbonylation-triggered radical relay rearrangement reaction in which the CO insertion step enables (hetero)aryl group migration.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


