网络结构、化学体系结构和诊断类别对认知地形之间转换的贡献。
IF 26.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
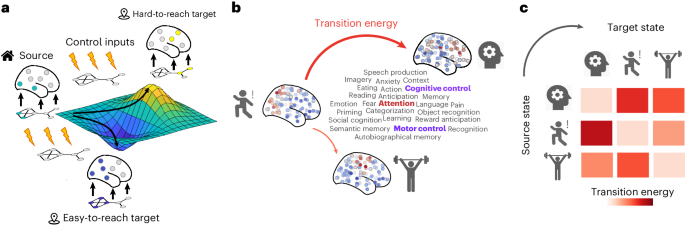
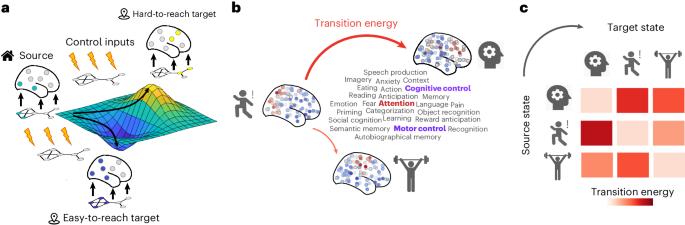
大脑网络结构与认知相关激活模式之间的联系机制在很大程度上仍不为人所知。在这里,我们利用网络控制原理,展示了人类连接组的结构是如何塑造来自 NeuroSynth 元分析数据库的 123 个实验定义的认知激活图谱(认知拓扑图)之间的转换的。具体来说,我们系统地整合了来自功能磁共振成像、弥散束成像、皮质形态测量和正电子发射断层扫描的大规模多模态神经成像数据,以模拟神经递质参与或皮质厚度变化如何重塑解剖学引导的认知状态之间的转换。我们的模型结合了神经递质受体密度图(18 种受体和转运体)以及与各种精神健康、神经变性、精神病和神经发育诊断类别有关的皮层厚度图(17,000 名患者和 22,000 名对照组)。研究结果提供了一个全面的查询表,描绘了大脑网络组织和化学结构如何相互作用以表现出不同的认知拓扑图,并为系统地确定促进认知拓扑图之间选择性转换的方法奠定了原则性基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Contributions of network structure, chemoarchitecture and diagnostic categories to transitions between cognitive topographies
The mechanisms linking the brain’s network structure to cognitively relevant activation patterns remain largely unknown. Here, by leveraging principles of network control, we show how the architecture of the human connectome shapes transitions between 123 experimentally defined cognitive activation maps (cognitive topographies) from the NeuroSynth meta-analytic database. Specifically, we systematically integrated large-scale multimodal neuroimaging data from functional magnetic resonance imaging, diffusion tractography, cortical morphometry and positron emission tomography to simulate how anatomically guided transitions between cognitive states can be reshaped by neurotransmitter engagement or by changes in cortical thickness. Our model incorporates neurotransmitter-receptor density maps (18 receptors and transporters) and maps of cortical thickness pertaining to a wide range of mental health, neurodegenerative, psychiatric and neurodevelopmental diagnostic categories (17,000 patients and 22,000 controls). The results provide a comprehensive look-up table charting how brain network organization and chemoarchitecture interact to manifest different cognitive topographies, and establish a principled foundation for the systematic identification of ways to promote selective transitions between cognitive topographies. An analysis of neuroimaging data across mental health, neurodegenerative, psychiatric and neurodevelopmental diagnostic categories highlights how brain network organization and chemoarchitecture interact to shape cognitive topographies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Biomedical Engineering
Medicine-Medicine (miscellaneous)
CiteScore
45.30
自引率
1.10%
发文量
138
期刊介绍:
Nature Biomedical Engineering is an online-only monthly journal that was launched in January 2017. It aims to publish original research, reviews, and commentary focusing on applied biomedicine and health technology. The journal targets a diverse audience, including life scientists who are involved in developing experimental or computational systems and methods to enhance our understanding of human physiology. It also covers biomedical researchers and engineers who are engaged in designing or optimizing therapies, assays, devices, or procedures for diagnosing or treating diseases. Additionally, clinicians, who make use of research outputs to evaluate patient health or administer therapy in various clinical settings and healthcare contexts, are also part of the target audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


