Nucleophosmin 1 通过支持线粒体氧化磷酸化和 ILC3 活性来促进粘膜免疫。
IF 27.7
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
Nucleophosmin 1(NPM1)在骨髓增生异常综合征(MDS)和急性髓性白血病中常发生突变。炎症性肠病(IBD)和骨髓增生异常综合征(MDS)并发的情况很常见,这表明 IBD 和 MDS 关系密切。在此,我们研究了 NPM1 在 IBD 和结肠炎相关性结直肠癌(CAC)中的功能。在 IBD 患者中,NPM1 的表达减少。与同窝对照组相比,Npm1+/-小鼠更易患急性结肠炎和实验诱发的CAC。Npm1 缺乏会损害产生白细胞介素-22(IL-22)的第三组先天性淋巴细胞(ILC3s)的功能。在ILC3s中缺乏Npm1的小鼠表现出IL-22生成减少和结肠炎发展加速。NPM1 对 ILC3s 中线粒体的生物生成和氧化磷酸化代谢非常重要。进一步的实验发现,NPM1 与 p65 合作促进 ILC3s 中线粒体转录因子 A(TFAM)的转录。在小鼠体内过量表达 NPM1 可增强 ILC3 的功能,并减轻葡聚糖硫酸钠诱导的结肠炎的严重程度。因此,我们的研究结果表明,ILC3 中的 NPM1 可通过 p65-TFAM 轴调节线粒体代谢,从而预防 IBD。本文章由计算机程序翻译,如有差异,请以英文原文为准。
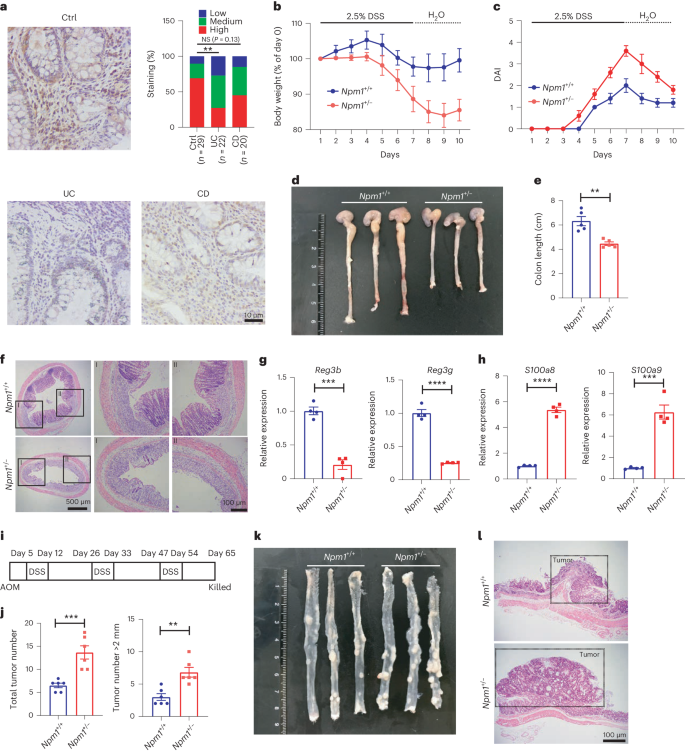
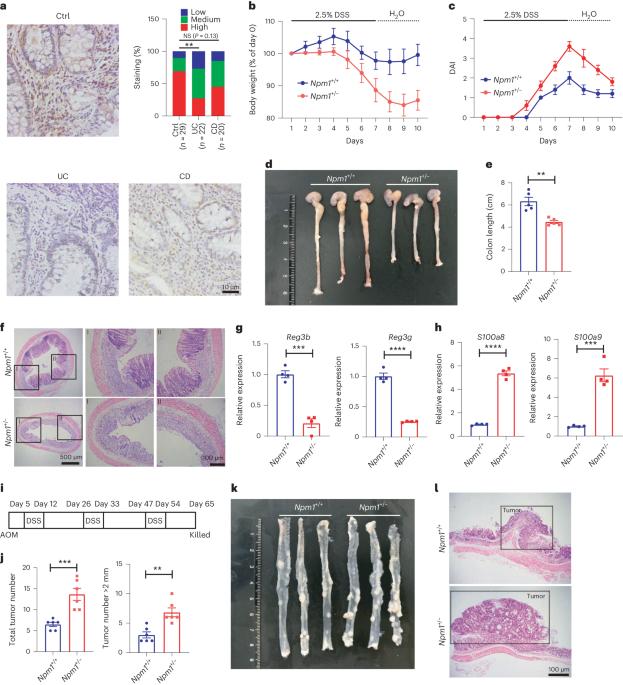
Nucleophosmin 1 promotes mucosal immunity by supporting mitochondrial oxidative phosphorylation and ILC3 activity
Nucleophosmin 1 (NPM1) is commonly mutated in myelodysplastic syndrome (MDS) and acute myeloid leukemia. Concurrent inflammatory bowel diseases (IBD) and MDS are common, indicating a close relationship between IBD and MDS. Here we examined the function of NPM1 in IBD and colitis-associated colorectal cancer (CAC). NPM1 expression was reduced in patients with IBD. Npm1+/− mice were more susceptible to acute colitis and experimentally induced CAC than littermate controls. Npm1 deficiency impaired the function of interleukin-22 (IL-22)-producing group three innate lymphoid cells (ILC3s). Mice lacking Npm1 in ILC3s exhibited decreased IL-22 production and accelerated development of colitis. NPM1 was important for mitochondrial biogenesis and metabolism by oxidative phosphorylation in ILC3s. Further experiments revealed that NPM1 cooperates with p65 to promote mitochondrial transcription factor A (TFAM) transcription in ILC3s. Overexpression of Npm1 in mice enhanced ILC3 function and reduced the severity of dextran sulfate sodium-induced colitis. Thus, our findings indicate that NPM1 in ILC3s protects against IBD by regulating mitochondrial metabolism through a p65-TFAM axis. Given associations between colitis and myelodysplastic syndrome (in which nucleophosmin 1 is often mutated), the authors here look at the contribution of nucleophosmin 1 to colitis, showing that it is important for protection mediated by ILC3s owing to effects on mitochondrial metabolism.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


