p16 依赖性 PD-L1 稳定性的增加可调节衰老细胞的免疫监视。
IF 17.3
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
衰老细胞的积累会促进衰老和与年龄有关的疾病,但衰老细胞逃避免疫清除并在组织中积累的分子机制仍有待阐明。在这里,我们报告了 p16 阳性衰老细胞上调免疫检查点蛋白程序性死亡配体 1(PD-L1),从而在衰老和慢性炎症中积聚。我们发现,p16 介导的细胞周期激酶 CDK4/6 抑制通过下调泛素依赖性降解诱导 PD-L1 在衰老细胞中的稳定性。激活效应细胞上 Fcγ 受体的抗 PD-L1 抗体可清除 PD-L1 和 p16 阳性细胞。我们的研究揭示了衰老细胞中 p16 依赖性调控 PD-L1 蛋白稳定性的分子机制,并揭示了靶向 PD-L1 改善衰老细胞免疫监视和改善衰老相关炎症的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
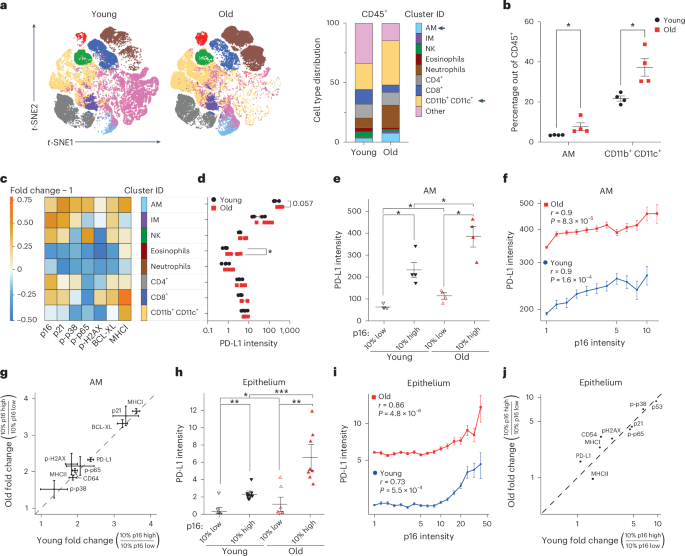
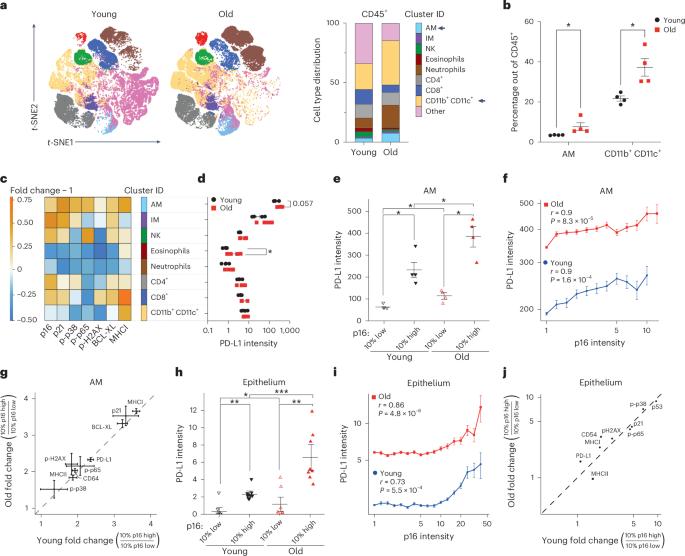
p16-dependent increase of PD-L1 stability regulates immunosurveillance of senescent cells
The accumulation of senescent cells promotes ageing and age-related diseases, but molecular mechanisms that senescent cells use to evade immune clearance and accumulate in tissues remain to be elucidated. Here we report that p16-positive senescent cells upregulate the immune checkpoint protein programmed death-ligand 1 (PD-L1) to accumulate in ageing and chronic inflammation. We show that p16-mediated inhibition of cell cycle kinases CDK4/6 induces PD-L1 stability in senescent cells via downregulation of its ubiquitin-dependent degradation. p16-expressing senescent alveolar macrophages elevate PD-L1 to promote an immunosuppressive environment that can contribute to an increased burden of senescent cells. Treatment with activating anti-PD-L1 antibodies engaging Fcγ receptors on effector cells leads to the elimination of PD-L1 and p16-positive cells. Our study uncovers a molecular mechanism of p16-dependent regulation of PD-L1 protein stability in senescent cells and reveals the potential of targeting PD-L1 to improve immunosurveillance of senescent cells and ameliorate senescence-associated inflammation. Majewska et al. show that p16-expressing senescent cells enhance the stability of the immune checkpoint PD-L1 by downregulating its proteasome-mediated ubiquitin-dependent degradation, leading to their accumulation in ageing and chronic inflammation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


