环烷基砜的对映体特异性交叉耦合。
IF 19.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
高效形成碳-碳键并控制立体化学的方法对于构建复杂分子至关重要。交叉偶联反应是构建分子最有效和最广泛使用的反应之一,而能够保留或安装手性的反应则是这一强大工具箱的最新成员。然而,自 1979 年首次使用砜类化合物以来,还没有使用砜类化合物进行对映选择性、对映特异性或对映转化性交叉偶联反应的实例。砜的高酸性使得人们不清楚这种转化在三级体系之外是否可行。在此,我们报告了环砜与格氏试剂的对映体特异性交叉偶联。尽管格氏试剂成分具有很强的碱性,但仍观察到高达 99% 的手性转移。原位监测显示,交叉偶联与竞争性去质子化具有动力学竞争性,从而实现了高度对映选择性转化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
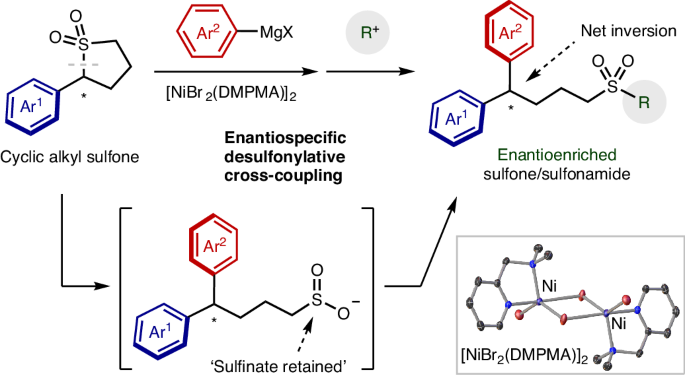
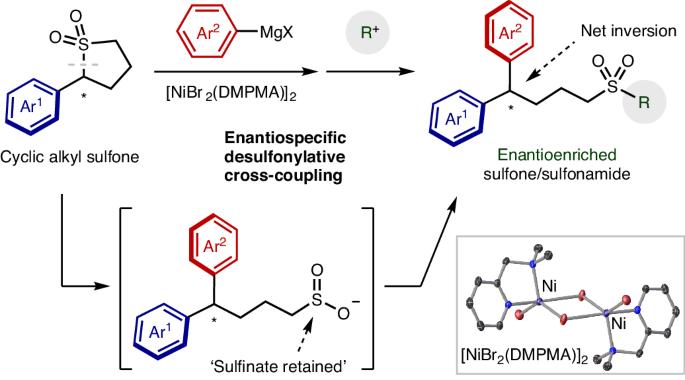
Enantiospecific cross-coupling of cyclic alkyl sulfones
Methods to form carbon–carbon bonds efficiently and with control of stereochemistry are critical for the construction of complex molecules. Cross-coupling reactions are among the most efficient and widely used reactions to construct molecules, with reactions enabling the retention or installation of chirality as recent additions to this powerful toolbox. Sulfones are robust, accessible organic electrophiles that have many attractive features as cross-coupling partners; however, since the first example of their use in 1979, there have been no examples of their use in enantioselective, enantiospecific or entantioconvergent cross-couplings. The high acidity of sulfones makes it unclear whether this transformation is even possible outside tertiary systems. Here we report the enantiospecific cross-coupling of cyclic sulfones and Grignard reagents. Up to 99% chirality transfer is observed despite the strong basicity of the Grignard components. In situ monitoring reveals that the cross-coupling is kinetically competitive with competing deprotonation, resulting in a highly enantioselective transformation. Cross-coupling reactions are among the most important carbon–carbon bond-forming reactions. Now the nickel-catalysed cross-coupling of chiral sulfones with Grignard reagents has been achieved with up to 99% retention of chirality. The speed of the cross-coupling relative to sulfone deprotonation and racemization is critical to enabling this enantiospecific process.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


