载脂蛋白 E 缺乏会通过增加铁含量导致脾巨噬细胞向 M1 表型极化。
IF 4.5
3区 医学
Q1 GENETICS & HEREDITY
引用次数: 0
摘要
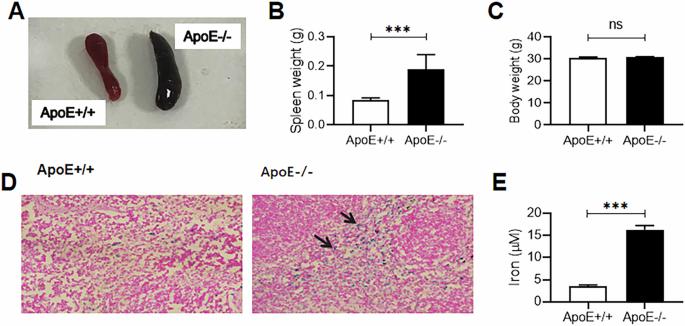
载脂蛋白 E(ApoE)在体内铁平衡中起着至关重要的作用,而巨噬细胞是哺乳动物体内负责处理铁的主要细胞。然而,载脂蛋白 E 是否会影响脾脏巨噬细胞(SM)的功能亚型和铁处理能力尚不清楚。在这里,我们研究了载脂蛋白E缺乏(ApoE-/-)对脾巨噬细胞极化和铁含量的影响及其潜在机制。研究发现,载脂蛋白E-/-诱导28周龄小鼠脾脏巨噬细胞中M1标记基因CD86、IL-1β、IL-6、IL-12、TNF-α和iNOS的表达显著增加,M2标记基因CD206、Arg-1、IL-10和Ym-1的表达减少、同时,ApoE-/-导致28周龄小鼠脾脏和/或SM中铁含量和铁蛋白、转铁蛋白受体1(TfR1)、铁调节蛋白1(IRP1)和血红素加氧酶1(HO-1)的表达显著增加,铁蛋白1(Fpn1)减少。结论是载脂蛋白E-/-可通过TfR/IRPs介导的铁摄取增加和Fpn1介导的铁释放减少来增加铁含量,从而导致SM极化为M1表型。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Apolipoprotein E deficiency leads to the polarization of splenic macrophages towards M1 phenotype by increasing iron content
Apolipoprotein E (ApoE) plays a crucial role in iron homeostasis in the body, while macrophages are the principal cells responsible for handling iron in mammals. However, it is unknown whether ApoE can affect the functional subtypes and the iron handling capacity of splenic macrophages (SM). Here, we investigated the effects of ApoE deficiency (ApoE−/−) on the polarization and iron content of SM and its potential mechanisms. ApoE−/− was found to induce a significant increase in the expressions of M1 marker genes CD86, IL-1β, IL-6, IL-12, TNF-α and iNOS and a reduction in M2 marker genes CD206, Arg-1, IL-10 and Ym-1 in SM of mice aged 28 weeks, Meanwhile, ApoE−/− caused a significant increase in iron content and expression of ferritin, transferrin receptor 1 (TfR1), iron regulatory protein 1 (IRP1) and heme oxygenase-1 (HO-1) and a reduction in ferroportin1 (Fpn1) in spleen and/or SM of mice aged 28 weeks. It was concluded that ApoE−/− can increase iron content through increased iron uptake mediated by TfR/ IRPs and decreased iron release mediated by Fpn1, leading to polarization of the SM to M1 phenotype.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Genes and immunity
医学-免疫学
CiteScore
8.90
自引率
4.00%
发文量
28
审稿时长
6-12 weeks
期刊介绍:
Genes & Immunity emphasizes studies investigating how genetic, genomic and functional variations affect immune cells and the immune system, and associated processes in the regulation of health and disease. It further highlights articles on the transcriptional and posttranslational control of gene products involved in signaling pathways regulating immune cells, and protective and destructive immune responses.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


