用于肿瘤细胞特异性代谢治疗的人工代谢酶
IF 34.9
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
代谢失调是癌症进展的一个关键特征。具有多个金属活性位点的酶在这一过程中发挥着重要作用。在这里,我们报告了首个类似代谢酶的 FeMoO4 纳米催化剂,被称为 "人工代谢酶"。它展示了双活性中心,即 Fe2+ 和四面体 Mo4+,反映了典型代谢酶黄嘌呤氧化还原酶的特征结构。利用空间动态代谢组学和肿瘤相关代谢物的评估,我们证明了FeMoO4代谢酶催化了肿瘤中大量黄嘌呤向尿酸的代谢转化。随后的代谢调整协调了与免疫细胞之间的串扰,这表明这是一种潜在的癌症治疗途径。我们的研究为癌症治疗引入了一种创新模式,即通过人工代谢酶的干预,对肿瘤细胞进行代谢重编程,使其自主调节并直接与免疫细胞对接,从而实现肿瘤细胞特异性代谢治疗。本文章由计算机程序翻译,如有差异,请以英文原文为准。
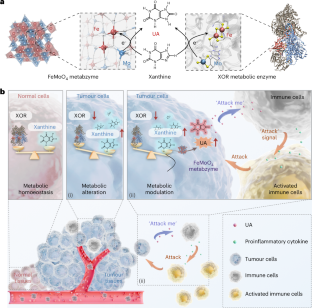
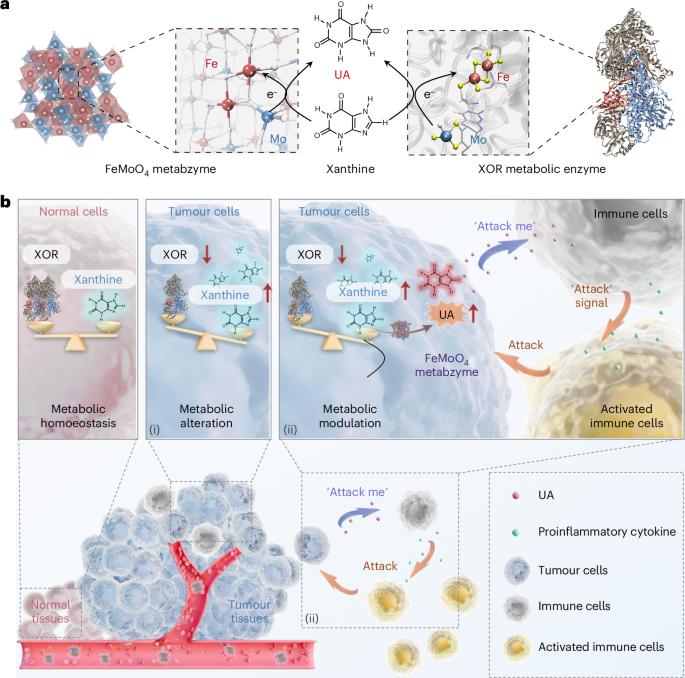
An artificial metabzyme for tumour-cell-specific metabolic therapy
Metabolic dysregulation constitutes a pivotal feature of cancer progression. Enzymes with multiple metal active sites play a major role in this process. Here we report the first metabolic-enzyme-like FeMoO4 nanocatalyst, dubbed ‘artificial metabzyme’. It showcases dual active centres, namely, Fe2+ and tetrahedral Mo4+, that mirror the characteristic architecture of the archetypal metabolic enzyme xanthine oxidoreductase. Employing spatially dynamic metabolomics in conjunction with the assessments of tumour-associated metabolites, we demonstrate that FeMoO4 metabzyme catalyses the metabolic conversion of tumour-abundant xanthine into uric acid. Subsequent metabolic adjustments orchestrate crosstalk with immune cells, suggesting a potential therapeutic pathway for cancer. Our study introduces an innovative paradigm in cancer therapy, where tumour cells are metabolically reprogrammed to autonomously modulate and directly interface with immune cells through the intervention of an artificial metabzyme, for tumour-cell-specific metabolic therapy. A metabolic-enzyme-like nanocatalyst is reported, dubbed ‘artificial metabzyme’. Tumour cells can be metabolically reprogrammed to autonomously modulate and interact with immune cells, facilitating tumour-cell-specific metabolic therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature nanotechnology
工程技术-材料科学:综合
CiteScore
59.70
自引率
0.80%
发文量
196
审稿时长
4-8 weeks
期刊介绍:
Nature Nanotechnology is a prestigious journal that publishes high-quality papers in various areas of nanoscience and nanotechnology. The journal focuses on the design, characterization, and production of structures, devices, and systems that manipulate and control materials at atomic, molecular, and macromolecular scales. It encompasses both bottom-up and top-down approaches, as well as their combinations.
Furthermore, Nature Nanotechnology fosters the exchange of ideas among researchers from diverse disciplines such as chemistry, physics, material science, biomedical research, engineering, and more. It promotes collaboration at the forefront of this multidisciplinary field. The journal covers a wide range of topics, from fundamental research in physics, chemistry, and biology, including computational work and simulations, to the development of innovative devices and technologies for various industrial sectors such as information technology, medicine, manufacturing, high-performance materials, energy, and environmental technologies. It includes coverage of organic, inorganic, and hybrid materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


