活动调控转录程序直接驱动突触发生
IF 21.2
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
摘要
尽管突触的分子组成和结构已被广泛探究,但人们对哪些基因程序直接激活突触基因表达以及如何调节突触基因表达却知之甚少。在这里,我们利用秀丽隐杆线虫多巴胺能神经元揭示了 EGL-43/MECOM 和 FOS-1/FOS 控制着一种依赖于活动的突触发生程序。任何一个因子的缺失都会严重降低突触前蛋白的表达。EGL-43和FOS-1可相互促进对方的表达,增加FOS-1与EGL-43基因座的结合亲和力可增加突触前蛋白的表达和突触功能。EGL-43 可调控多种转录因子的表达,包括活性调控因子和发育因子,这些因子可确定多巴胺能特性的多个方面。总之,我们描述了发育过程中活动调节突触形成的强大遗传程序。本文章由计算机程序翻译,如有差异,请以英文原文为准。
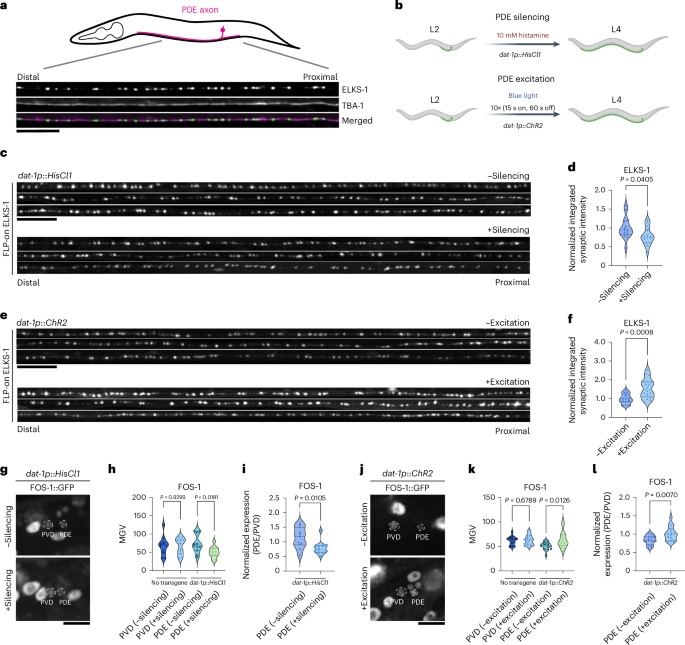
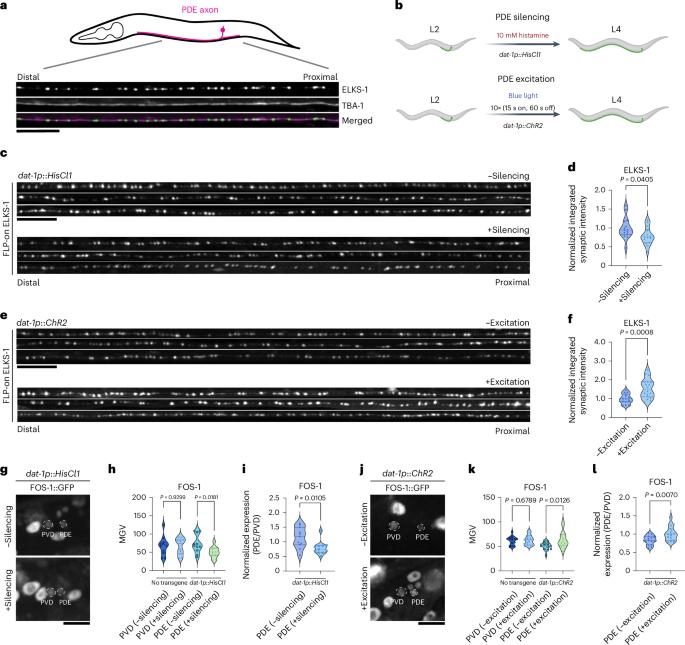
An activity-regulated transcriptional program directly drives synaptogenesis
Although the molecular composition and architecture of synapses have been widely explored, much less is known about what genetic programs directly activate synaptic gene expression and how they are modulated. Here, using Caenorhabditis elegans dopaminergic neurons, we reveal that EGL-43/MECOM and FOS-1/FOS control an activity-dependent synaptogenesis program. Loss of either factor severely reduces presynaptic protein expression. Both factors bind directly to promoters of synaptic genes and act together with CUT homeobox transcription factors to activate transcription. egl-43 and fos-1 mutually promote each other’s expression, and increasing the binding affinity of FOS-1 to the egl-43 locus results in increased presynaptic protein expression and synaptic function. EGL-43 regulates the expression of multiple transcription factors, including activity-regulated factors and developmental factors that define multiple aspects of dopaminergic identity. Together, we describe a robust genetic program underlying activity-regulated synapse formation during development. Neuronal activity contributes to synapse formation and plasticity. Here the authors demonstrate that activity stimulates developmental programs to directly modulate synapse formation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


