气道蛋白质组学揭示了泼尼松龙对mepolizumab治疗哮喘的广泛残余抗炎作用。
IF 11.4
1区 医学
Q1 ALLERGY
引用次数: 0
摘要
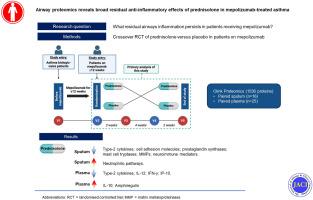
背景介绍美妥珠单抗是一种抗白细胞介素-5单克隆抗体,用于治疗严重嗜酸性粒细胞性哮喘(SEA),可减少哮喘加重。使用甲泼尼单抗后残留的气道炎症可能导致病情持续恶化。口服皮质类固醇仍是治疗这些残留加重症状的主要方法:我们的研究旨在探索甲泼尼单抗治疗后气道炎症对皮质类固醇的反应性,以发现 IL-5 通路以外的潜在可治疗炎症机制:MAPLE试验是一项多中心、随机、双盲、安慰剂对照、交叉研究,在稳定状态下对27名接受mepolizumab治疗的SEA患者进行为期2周的大剂量口服泼尼松龙治疗。我们使用高通量 Olink® 蛋白质组学分析了来自 MAPLE 试验的配对痰液(16 份)和血浆(25 份)样本。我们还使用 ELISA 分析了其他痰液蛋白质:结果:在接受mepolizumab治疗的患者中,泼尼松龙显著下调了与2型炎症和趋化有关的痰蛋白,包括IL-4、IL-5、IL-13、CCL24、CCL26、EDN、CCL17、CCL22、OX40受体、FCER2和ST2受体。泼尼松龙还能下调细胞粘附分子、前列腺素合成酶、肥大细胞胰蛋白酶、MMP1、MMP12 和神经免疫介质。中性粒细胞通路上调。血浆中的 2 型蛋白以及 IL-12、IFN-γ 和 IP-10 也出现下调。IL-10和安非他酮上调:结论:在稳定状态下,泼尼松龙在甲泼尼单抗的基础上具有广泛的抗炎作用。结论:在稳定状态下,泼尼松龙在美泊珠单抗的基础上具有广泛的抗炎作用,这些作用具有异质性,可能与残余病情加重有临床相关性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Airway proteomics reveals broad residual anti-inflammatory effects of prednisolone in mepolizumab-treated asthma
Background
Mepolizumab is an anti-IL-5 mAb treatment for severe eosinophilic asthma that reduces asthma exacerbations. Residual airway inflammation with mepolizumab therapy may lead to persistent exacerbations. Oral corticosteroids remain the main treatment for these residual exacerbations.
Objective
Our study aimed to explore the corticosteroid responsiveness of airway inflammation after mepolizumab treatment to find potentially treatable inflammatory mechanisms beyond the IL-5 pathway.
Methods
The MAPLE trial was a multicenter, randomized, double-blind, placebo-controlled, crossover study of 2 weeks of high-dose oral prednisolone treatment at stable state in 27 patients treated with mepolizumab for severe eosinophilic asthma. We analyzed paired sputum (n = 16) and plasma (n = 25) samples from the MAPLE trial using high-throughput Olink proteomics. We analyzed additional sputum proteins using ELISA.
Results
In patients receiving mepolizumab, prednisolone significantly downregulated sputum proteins related to type 2 inflammation and chemotaxis including IL-4, IL-5, IL-13, CCL24, CCL26, EDN, CCL17, CCL22, OX40 receptor, FCER2, and the ST2 receptor. Prednisolone also downregulated cell adhesion molecules, prostaglandin synthases, mast cell tryptases, MMP1, MMP12, and neuroimmune mediators. Neutrophilic pathways were upregulated. Type 2 proteins were also downregulated in plasma, combined with IL-12, IFN-γ, and IP-10. IL-10 and amphiregulin were upregulated.
Conclusions
At stable state, prednisolone has broad anti-inflammatory effects on top of mepolizumab. These effects are heterogeneous and may be clinically relevant in residual exacerbations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
25.90
自引率
7.70%
发文量
1302
审稿时长
38 days
期刊介绍:
The Journal of Allergy and Clinical Immunology is a prestigious publication that features groundbreaking research in the fields of Allergy, Asthma, and Immunology. This influential journal publishes high-impact research papers that explore various topics, including asthma, food allergy, allergic rhinitis, atopic dermatitis, primary immune deficiencies, occupational and environmental allergy, and other allergic and immunologic diseases. The articles not only report on clinical trials and mechanistic studies but also provide insights into novel therapies, underlying mechanisms, and important discoveries that contribute to our understanding of these diseases. By sharing this valuable information, the journal aims to enhance the diagnosis and management of patients in the future.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


