使用扭曲碳纳米带对氨基酸进行手性识别:DFT 研究
IF 5.5
2区 材料科学
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
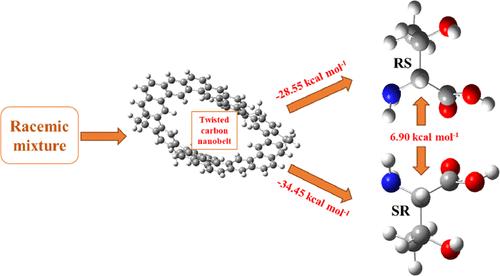
除了化学领域之外,分子手性以及对称性、不对称性、手性、对称性破坏和手性识别等相关概念在科学领域的影响也越来越大。本研究利用密度泛函理论研究了扭曲碳纳米带(TCNB)对氨基酸(AA)的手性识别。相互作用能(Eint)的值范围为 -28.55 至 -34.45 kcal mol-1。Eint 有两种不同的趋势,TCNB 对脯氨酸的 R 手性异构体和组氨酸的 S 手性异构体具有选择性。同样,对于苏氨酸,TCNB 对 SR 苏氨酸具有选择性。苏氨酸@TCNB(SR 和 RS)对映体复合物的手性识别能最为明显,即 6.90 kcal mol-1。分子中原子量子理论(QTAIM)和非共价相互作用(NCI)分析表明,与 R 对映体相比,S 对映体在每种情况下都具有最大的相互作用。电子密度差(EDD)和天然键轨道(NBO)分析表明,电荷从氨基酸向带转移,RS-thre@TCNB 的电荷转移最大,为 2.260(e-)。前沿分子轨道(FMO)分析表明,络合时能隙下降。R-pro@TCNB 的能隙下降幅度最大(从 3.59 eV 下降到 3.42 eV),这表明 TCNB 对脯氨酸具有很高的选择性。目前的研究凸显了 TCNB 对手性分子的选择性,显示出对氨基酸的 S 和 R 对映体具有显著的手性鉴别能力。这项工作有助于深入了解扭曲碳纳米带的分子相互作用和手性识别。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Chiral Discrimination of Amino Acids by Using a Twisted Carbon Nanobelt: A DFT Study
Beyond the confines of chemistry, molecular chirality and related ideas like symmetry, asymmetry, handedness, symmetry breaking, and chiral recognition have a growing influence in science. In the current study, a twisted carbon nanobelt (TCNB) is used for the chiral discrimination of amino acids (AA) using density functional theory study. The values of interaction energy (Eint) range from −28.55 to −34.45 kcal mol–1. Two distinct trends of Eint are identified, with the TCNB demonstrating selectivity for R-enantiomers of proline and S-enantiomers of histidine. Similarly, for threonine, the TCNB is selective toward SR-threonine. Chiral discrimination energy is most pronounced for threonine@TCNB (SR and RS) enantiomeric complexes, i.e., 6.90 kcal mol–1. Quantum theory of atoms in molecules (QTAIM) and noncovalent interaction (NCI) analyses reveal that the S enantiomer in each case has maximum interactions compared to the R enantiomer. Electron density difference (EDD) and natural bond orbital (NBO) analyses indicate charge transfer from amino acids toward the belt, with RS-thre@TCNB having a maximum charge transfer, i.e., 2.260 (e–). Frontier molecular orbital (FMO) analysis reveals a decline in energy gap upon complexations. The highest decrease in energy gap is seen for R-pro@TCNB (3.42 eV from 3.59 eV), which displays high selectivity of the TCNB toward proline. The current study highlights the selectivity of the TCNB toward chiral molecules, showing a significant chiral discrimination ability for S and R enantiomers of amino acids. This work contributes valuable insights into the molecular interactions and chiral recognition involving twisted carbon nanobelts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Nano Materials
Multiple-
CiteScore
8.30
自引率
3.40%
发文量
1601
期刊介绍:
ACS Applied Nano Materials is an interdisciplinary journal publishing original research covering all aspects of engineering, chemistry, physics and biology relevant to applications of nanomaterials. The journal is devoted to reports of new and original experimental and theoretical research of an applied nature that integrate knowledge in the areas of materials, engineering, physics, bioscience, and chemistry into important applications of nanomaterials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


